Abstract.
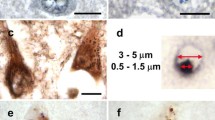
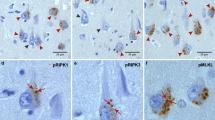
Granulovacuolar degeneration (GVD) is a diagnostic neuropathological feature of Alzheimer's disease (AD). In some neurons, apoptosis has been hypothesized to be a primary mechanism causing neuronal cell death in AD. In this study we investigated CA1 neurons with GVD in AD and Down's syndrome (DS) brain. We demonstrated that activated caspase-3 and a caspase-cleaved cleavage product of the amyloid precursor protein (cAPP) are co-localized in GVD granules, and that these same cells often show nuclear DNA damage. In contrast, activated caspase-8 is present in the cytoplasm but not within the granules of GVD neurons. A caspase-cleavage product of fodrin that accumulates in many AD and DS neurons is not present in GVD granules. These data support a role for the activation of apoptotic mechanisms in selective compartments exhibiting GVD.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Su, J.H., Kesslak, P.J., Head, E. et al. Caspase-cleaved amyloid precursor protein and activated caspase-3 are co-localized in the granules of granulovacuolar degeneration in Alzheimer's disease and Down's syndrome brain. Acta Neuropathol 104, 1–6 (2002). https://doi.org/10.1007/s00401-002-0548-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-002-0548-2