Abstract
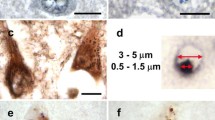
Alzheimer’s Disease (AD) is characterized by the appearance of neurofibrillary and granulovacuolar lesions in the brains of affected individuals. The former is composed of hyperphosphorylated aggregates of the microtubule-associated protein tau. The latter is poorly characterized but reacts strongly with anti-phosphoepitope antibodies indicating that it too accumulates phosphoproteins. Both lesions react strongly with antibodies directed against members of the casein kinase-1 family of phosphotransferases, a group of closely related protein kinases that frequently function in tandem with the ubiquitin modification system. To determine whether individual members of the casein kinase-1 family differentially associate with AD lesions, hippocampal sections isolated from late stage cases of AD were subjected to double-label fluorescence immunohistochemistry using a panel of selective anti-casein kinase 1 antibodies and small-molecule fluorochromes thioflavin S and thiazin red. The resultant colocalization patterns revealed that the alpha CK1 isoform strongly correlated with thioflavin S and thiazin red fluorescence, indicating that it preferentially associated with neurofibrillary lesions. In contrast, the delta isoform staining pattern was dominated by colocalization with granulovacuolar degeneration bodies. These findings suggest that granulovacuolar and neurofibrillary lesions occupy separate populations of neurons, and implicate CK1 isoforms in the generation of lesion-associated phosphoepitopes. They also suggest a nexus between the phosphorylation and ubiquitination modifications found in both lesions.





Similar content being viewed by others
References
Abdel-Sater F, El Bakkoury M, Urrestarazu A, Vissers S, Andre B (2004) Amino acid signaling in yeast: casein kinase I and the Ssy5 endoprotease are key determinants of endoproteolytic activation of the membrane-bound Stp1 transcription factor. Mol Cell Biol 24:9771–9785
Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I (2002) Axin-mediated CKI phosphorylation of b-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 16:1066–1076
Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol (Berl) 37:111–118
Ball MJ (1978) Topographic distribution of neurofibrillary tangles and granulovacuolar degeneration in hippocampal cortex of aging and demented patients. A quantitative study. Acta Neuropathol (Berl) 42:73–80
Ball MJ, Nuttall K (1981) Topography of neurofibrillary tangles and granulovacuoles in hippocampi of patients with Down’s syndrome: quantitative comparison with normal ageing and Alzheimer’s disease. Neuropathol Appl Neurobiol 7:13–20
Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res 477:90–99
Baum L, Hansen L, Masliah E, Saitoh T (1996) Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol Chem Neuropathol 29:253–261
Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11:153–163
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Braak E, Braak H, Mandelkow EM (1994) A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol (Berl) 87:554–567
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10:1089–1099
Bruns G, Beerhalter H (1955) Dye analysis. The fluorochrome mixture, thioflavine S. Acta Histochem 1:254–271
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33:95–130
Campbell HD, Webb GC, Fountain S, Young IG (1997) The human PIN1 peptidyl-prolyl cis/trans isomerase gene maps to human chromosome 19p13 and the closely related PIN1L gene to 1p31. Genomics 44:157–162
Carmel G, Mager EM, Binder LI, Kuret J (1996) The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem 271:32789–32795
Cho JH, Johnson GV (2003) Glycogen synthase kinase 3b phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J Biol Chem 278:187–193
Dickson DW, Ksiezak-Reding H, Davies P, Yen SH (1987) A monoclonal antibody that recognizes a phosphorylated epitope in Alzheimer neurofibrillary tangles, neurofilaments and tau proteins immunostains granulovacuolar degeneration. Acta Neuropathol 73:254–258
Eide EJ, Vielhaber EL, Hinz WA, Virshup DM (2002) The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Ie. J Biol Chem 277:17248–17254
Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F, Vielhaber EL, Giovanni A, Virshup DM (2005) Control of mammalian circadian rhythm by CKIe-regulated proteasome-mediated PER2 degradation. Mol Cell Biol 25:2795–2807
Evers P, Uylings HB, Suurmeijer AJ (1998) Antigen retrieval in formaldehyde-fixed human brain tissue. Methods 15:133–140
Feng Y, Davis NG (2000) Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol Cell Biol 20:5350–5259
Fiol CJ, Wang A, Roeske RW, Roach PJ (1990) Ordered multisite protein phosphorylation. Analysis of glycogen synthase kinase 3 action using model peptide substrates. J Biol Chem 265:6061–6065
Galvan M, David J, Delacourte A, Luna J, Mena R (2001) Sequence of neurofibrillary changes in aging and Alzheimer’s disease: a confocal study with phospho-tau antibody, AD2. J Alzheimers Dis 3:417–425
Garcia-Sierra F, Wischik CM, Harrington CR, Luna-Munoz J, Mena R (2001) Accumulation of C-terminally truncated tau protein associated with vulnerability of the perforant pathway in early stages of neurofibrillary pathology in Alzheimer’s disease. J Chem Neuroanat 22:65–77
Ghanevati M, Miller CA (2005) Phospho-b-catenin accumulation in Alzheimer’s disease and in aggresomes attributable to proteasome dysfunction. J Mol Neurosci 25:79–94
Ghoshal N, Smiley JF, DeMaggio AJ, Hoekstra MF, Cochran EJ, Binder LI, Kuret J (1999) A new molecular link between the fibrillar and granulovacuolar lesions of Alzheimer’s disease. Am J Pathol 155:1163–1172
Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI (2002) Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol 177:475–493
Hicke L, Zanolari B, Riezman H (1998) Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J Cell Biol 141:349–358
Holzer M, Gartner U, Stobe A, Hartig W, Gruschka H, Bruckner MK, Arendt T (2002) Inverse association of Pin1 and tau accumulation in Alzheimer’s disease hippocampus. Acta Neuropathol (Berl) 104:471–481
Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M (2005) The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal 17:675–689
Kondratick CM, Vandre DD (1996) Alzheimer’s disease neurofibrillary tangles contain mitosis-specific phosphoepitopes. J Neurochem 67:2405–2416
Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I (1993) Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268:24374–24384
Ksiezak-Reding H, Liu WK, Yen SH (1992) Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res 597:209–219
Kuret J, Johnson GS, Cha D, Christenson ER, DeMaggio AJ, Hoekstra MF (1997) Casein kinase 1 is tightly associated with paired-helical filaments isolated from Alzheimer’s disease brain. J Neurochem 69:2506–2515
LaGrassa TJ, Ungermann C (2005) The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol 168:401–414
Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion JP (2002) The active form of glycogen synthase kinase-3beta is associated with granulovacuolar degeneration in neurons in Alzheimer’s disease. Acta Neuropathol (Berl) 103:91–99
Li G, Yin H, Kuret J (2004) Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J Biol Chem 279:15938–15945
Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP (1999) The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399:784–788
Lübke U, Mercken M, Vandermeeren M, Ceuterick-de Groote C, Vanmechelen E, Martin J-J, Cras P (1993) Comparative study of granulovacuolar degeneration in neurodegenerative diseases. In: Nicolini M (eds) Alzheimer’s disease and related disorders: communications. Pergamon, Oxford, pp 149–150
Marchal C, Haguenauer-Tsapis R, Urban-Grimal D (2000) Casein kinase I-dependent phosphorylation within a PEST sequence and ubiquitination at nearby lysines signal endocytosis of yeast uracil permease. J Biol Chem 275:23608–23614
Margittai M, Langen R (2004) Template-assisted filament growth by parallel stacking of tau. Proc Natl Acad Sci USA 101:10278–10283
McKay RM, Peters JM, Graff JM (2001) The casein kinase I family in Wnt signaling. Dev Biol 235:388–396
Mena R, Edwards P, Perez-Olvera O, Wischik CM (1995) Monitoring pathological assembly of tau and beta-amyloid proteins in Alzheimer’s disease. Acta Neuropathol (Berl) 89:50–56
Mirra SS, Hart MN, Terry RD (1993) Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med 117:132–144
Necula M, Kuret J (2004) Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J Biol Chem 279:49694–49703
Newcombe RG (1998) Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 17:2635–2650
Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA 102:6990–6995
Okamoto K, Hirai S, Iizuka T, Yanagisawa T, Watanabe M (1991) Reexamination of granulovacuolar degeneration. Acta Neuropathol 82:340–345
Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I (1997) Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 56:70–78
Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF (1999) Distribution of active glycogen synthase kinase 3b (GSK-3b) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 58:1010–1019
Romijn HJ, van Uum JF, Breedijk I, Emmering J, Radu I, Pool CW (1999) Double immunolabeling of neuropeptides in the human hypothalamus as analyzed by confocal laser scanning fluorescence microscopy. J Histochem Cytochem 47:229–236
Schnell SA, Staines WA, Wessendorf MW (1999) Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 47:719–730
Schwab C, DeMaggio AJ, Ghoshal N, Binder LI, Kuret J, McGeer PL (2000) Casein kinase 1 delta is associated with pathological accumulation of tau in several neurodegenerative diseases. Neurobiol Aging 21:503–510
Spielewoy N, Flick K, Kalashnikova TI, Walker JR, Wittenberg C (2004) Regulation and recognition of SCFGrr1 targets in the glucose and amino acid signaling pathways. Mol Cell Biol 24:8994–9005
Sun A, Nguyen XV, Bing G (2002) Comparative analysis of an improved thioflavin-s stain, Gallyas silver stain, and immunohistochemistry for neurofibrillary tangle demonstration on the same sections. J Histochem Cytochem 50:463–472
Sun B, Chen L, Cao W, Roth AF, Davis NG (2004) The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXPhi adaptin sorting signal. Mol Biol Cell 15:1397–1406
Vancura A, Sessler A, Leichus B, Kuret J (1994) A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J Biol Chem 269:19271–19278
Vincent I, Zheng JH, Dickson DW, Kress Y, Davies P (1998) Mitotic phosphoepitopes precede paired helical filaments in Alzheimer’s disease. Neurobiol Aging 19:287–296
Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C (2001) Phosphorylation regulates intracellular trafficking of b-secretase. J Biol Chem 276:14634–14641
Wang X, Hoekstra MF, DeMaggio AJ, Dhillon N, Vancura A, Kuret J, Johnston GC, Singer RA (1996) Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol Cell Biol 16:5375–5385
Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, Jiang J (2005) Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell 8:267–278
Acknowledgements
We thank Profs. L.I. Binder and R.W. Berry (Northwestern University, Il) for guidance with immunostaining, and to Prof. Paul D. Coleman and Dr. Linda M. Callahan (University of Rochester, NY) for generous access to tissue. We also thank Prof. Richard Burry, Ms. Kathy Wolken and Mr. Brian Kemmenoe (Campus Microscopic and Imaging facility, Ohio State University, OH) for guidance with confocal microscopy. This work was supported by NIH grants AG14452 (J.K.), AG16756 (D.D.V.), MH/NS31862 (Harvard Brain Tissue Resource Center), AG018254 (Rochester AD Core Center), and AG010161 (Rush AD Core Center).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kannanayakal, T.J., Tao, H., Vandre, D.D. et al. Casein kinase-1 isoforms differentially associate with neurofibrillary and granulovacuolar degeneration lesions. Acta Neuropathol 111, 413–421 (2006). https://doi.org/10.1007/s00401-006-0049-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0049-9