Abstract
Metabolic bone disorders and associated fragility fractures are major causes of disability and mortality worldwide and place an important financial burden on the global health systems. These disorders result from an unbalance between bone anabolic and resorptive processes and are characterized by different pathophysiological mechanisms. Drugs are available to treat bone metabolic pathologies, but they are either poorly effective or associated with undesired side effects that limit their use. The molecular mechanism underlying the most common metabolic bone disorders, and the availability, efficacy, and limitations of therapeutic options currently available are discussed here. A source for the unmet need of novel drugs to treat metabolic bone disorders is marine organisms, which produce natural osteoactive compounds of high pharmaceutical potential. In this review, we have inventoried the marine osteoactive compounds (MOCs) currently identified and spotted the groups of marine organisms with potential for MOC production. Finally, we briefly examine the availability of in vivo screening and validation tools for the study of MOCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic bone disorders (MBDs) pose a significant global health challenge, with fragility fractures affecting a substantial portion of the population, notably among the elderly [1]. At the heart of fragility fractures lies the disruption of bone remodeling, an essential homeostatic process that involves the removal of old or damaged bone, followed by the deposition of new bone [2]. In the first part of this review, MBDs are described according to their impact on bone mineral density (BMD), a physiological parameter of bone health with clinical relevance [3]. Since the pathophysiological mechanisms that underlie changes in BMD are many and various, we also provide a detailed analysis of the molecular mechanisms underpinning the most common MBDs. This section also reviews the therapeutic strategies currently available for treating MBDs, assessing their efficacy and limitations, and outlines emerging pharmaceutical options. The second part of this review intends to shed some light on the potential of marine osteoactive compounds (MOCs) as natural drugs to treat MBDs. It goes through the remarkable diversity of sourced organisms and identified compounds, and gives some insights on the molecular mechanisms underlying MOC action and on drug development status. The final part of this review underscores the need for coordinated efforts between chemical characterization and the implementation of screening tools already available to explore marine organism biodiversity for bone anabolic and/or antiresorptive bioactives.
The burden of metabolic bone disorders
In 2019, a meta-analysis of available data from 204 countries and territories reported a global incidence of fragility fractures around 2.3% of the total population and 15.4% of the elderly sub-population [1]. Bone fragility is a major concern for the global health system, causing severe disability and mortality worldwide, and placing an important financial burden on the society [4]. At the origin of fragility fractures is the dysregulation of a fundamental homeostatic process: bone remodeling. To maintain mechanical properties and architectural integrity throughout life, bone must renew senescent and damaged structures through a process requiring the concerted resorption and formation of bone mineralized matrix. An unbalance between these two processes will prompt metabolic bone disorders [2]. As different pathologies are characterized by different causing mechanisms, we will start this review with a brief description of the mineral phenotypes and molecular mechanisms underlying such disorders.
Molecular mechanisms of metabolic bone disorders
Bone mineral density (BMD), defined as “the amount of mineral per cubic centimeter of bone tissue”, represents the gold standard in clinical practice to establish a pathological alteration of mineral content and identify patients with MBDs. Based on this clinical marker, low-BMD pathologies include osteomalacia [5], nutritional rickets [6], osteopenia, and osteoporosis [7], while high-BMD pathologies are genetic disorders united under the term osteopetrosis [8]. Finally, Paget’s disease of bone [9], primary hyperparathyroidism [10], and renal osteodystrophy [11] can be considered BMD-independent pathologies, as it has been demonstrated that they are not unequivocally diagnosed by a reduced BMD, and several manifestations of these disorders are characterized by locally elevated BMD. Although this functional classification of MBDs may be appropriate in a diagnostic setting, the therapeutic approaches adopted will mostly depend on the pathophysiological mechanisms at the origin of the disease. As such, in the following section, we have further classified bone disorders into (i) disorders affecting the mineral homeostasis through the vitamin D (VD)–parathyroid hormone (PTH) regulatory network, (ii) disorders caused by an excessive osteoclast function, and (iii) disorders induced by a defective osteoclast function.
Disorders resulting from an altered mineral homeostasis
Osteomalacia and rickets are primarily caused by calcium or VD deficiency in adults and children, respectively [12]. Causes of these deficiencies are vast, e.g., reduced dietary intake, malabsorption in patients with gastrointestinal or liver disorders, or increased excretion induced by nephropathologies [3]. Low levels of these essential nutrients drive the mineral homeostatic system to change the source of circulating calcium from intestinal absorption to bone resorption. In this situation, PTH stimulates osteoclast differentiation by inducing an overproduction of RANKL (receptor activator of nuclear factor kappa-B ligand) and M-CSF (macrophage colony-stimulating factor) by osteoblasts, osteocytes, bone marrow stromal cells, and resident lymphocytes [13]. The persistency of this condition leads to osteopenic bones in adults and bended bones in children [14]. Osteomalacia can be rescued in adults upon VD and calcium supplementation, but bone deformities in rachitic children are often irreversible and can only be treated by surgery [15].
Primary hyperparathyroidism is an endocrine disorder characterized by hypercalcemia (elevated blood calcium levels) and inappropriate PTH levels, caused by benign or cancerous tumors in parathyroid glands [16]. Skeletal phenotype is characterized by loss of cortical bone, reduced BMD leading to osteopenia, and an increased risk of fracture in both vertebral and appendicular sites [17]. In the absence of suitable drugs, the only efficient cure is the surgical removal of parathyroid tissue or glands (parathyroidectomy). If surgery is not an option, a blend of calcium regulating agents, bone anabolic, and antiresorptive drugs may be used [16].
Renal osteodystrophy is a condition that covers skeletal disorders in patients suffering from chronic kidney disease (CKD), e.g., osteoporosis, osteomalacia, osteitis fibrosa, and adynamic bone disease [18, 19]. Initially, renal insufficiency triggers a retention of phosphorous and an accumulation of uremic toxins in blood, inducing a state of low bone metabolism known as adynamic bone disease [18]. This condition may result from the acquisition of a PTH signaling resistance by the bone tissue. The persistency of the adynamic bone condition, high level of phosphorous, and reduced circulating calcitriol (1,25-hydroxyvitamin D3) induces hypocalcemia and stimulates parathyroid glands, exacerbating the quantity of PTH in the serum. Patients eventually develop secondary hyperparathyroidism [19], whose histological landmarks are defined as osteitis fibrosa, which is characterized by an increased bone turnover, increased osteoblast number and activity, woven osteoid, increased osteoclast number and activity, overall increased bone resorption, low BMD, and increased fragility [18, 19].
Disorders resulting from an excessive osteoclast activity
Osteoporosis (OP) and Paget’s disease of bone (PDB) are the most common MBDs, with a prevalence of 18.3% [20] and 0.6% [21], respectively, and both conditions result from a dysfunctional and overregulated bone resorption by osteoclasts [2]. PDB pathophysiology involves the increased formation of hyper-resorptive osteoclasts during the osteolytic and initial phase of the disease. In an attempt to recover the loss of bone mineral, the body increases bone formation, a compensatory mechanism which results in the production of an unorganized and woven bone matrix [22]. Typically, pagetic patients show a localized symptomatology (two forms—monostotic, affecting a single bone, and polyostotic, affecting more skeletal elements—exist) with a higher number of atypical osteoclasts characterized by a larger size, an increased number of nuclei, and an elevated resorptive activity. Osteoclast precursors are generally highly responsive to pro-osteoclastogenic signals such as RANKL and 1,25-(OH)2D3 and resistant to apoptotic signals [23, 24]. Clinical features of PDB include bone pain and increased serum alkaline phosphatase (ALP); microfractures and increased bone vascularization may also be observed [25], leading with time to deformations due to the weakened structure [23, 24]. Leading causes of PDB are still not fully understood, although it appears that bone formation, despite being rapid and unorganized, is in fact intrinsically normal [26]. Genetic factors associated to the disease include a plethora of mutations and variants in genes associated to osteoclast differentiation and activation, while environmental factors may include epigenetic factors, exposure to certain toxins, and infection by paramyxoviruses [27]. No cure exists for PDB, and therapeutic strategies currently available to alleviate disease symptoms focus on a set of antiresorptive drugs, mostly bisphosphonates, targeted at restoring normal levels of bone resorption. Anti-inflammatory drugs may also be implemented, as well as vitamin D and calcium supplementation, to prevent possible negative effects of the elevated bone resorption over parathyroid function, which may lead to secondary hyperparathyroidism.
Osteoporosis and osteopenia are also characterized by a dysregulated resorptive process. It is important to highlight that although we have classified osteoporosis as an “excessive osteoclast activity” disease, this disorder is characterized by a complex etiology and a variety of pathophysiological mechanisms and, in some cases, the imbalance in bone remodeling is caused by a reduced bone formation [28]. Four main pathophysiological mechanisms have been identified to be at the origin of osteoporosis, and these may overlap in some patients: postmenopausal osteoporosis, age-related osteoporosis, immobilization-induced osteoporosis, and drug-induced osteoporosis.
Postmenopausal osteoporosis (also known as primary osteoporosis) is a complex and multifactorial condition. In premenopausal women, estrogens participate in bone anabolism by inhibiting osteoblast [29] and osteocytes [30] apoptosis, thus increasing their life spam. Estrogens also prevent bone resorption by inhibiting RANKL-mediated osteoclastogenesis [31], stimulating the production of anti-osteoclastogenic cytokines by regulatory T cells [32], and inducing osteoblast-mediated osteoclast apoptosis in a paracrine manner [33]. Estrogens also excerpt a suppressive effect over thymic function, reducing the population of inflammatory T cells [34]. After the menopause, circulating estrogens are depleted as a result of reduced ovarian synthesis, and the suppressive effect they normally have over thymic function is diminished. As activated T cells produce pro-osteoclastogenic cytokines such as IL-1b and TNF-α [35], a chronically elevated bone remodeling is established at menopause, where bone resorption is not compensated by bone formation. This mechanism leads to an overall reduced BMD, increased fragility and fracture risk [36]. Age-related osteoporosis affects both woman and men and initiates after the peak of BMD at adolescence. Rate is similar in both genders but may be intensified in women entering menopause [37]. An hypothesis for a long time [38], there is now a growing body of evidence that support the role of an age-related increase in oxidative stress in the age-related diminution of BMD. In this scenario, reactive oxygen species (ROS) induce bone loss by stimulating osteoclast differentiation [39] and osteoblast apoptosis [40].
The term secondary osteoporosis is used for disorders where bone loss is a consequence of other conditions or medications [41]. It includes immobilization-induced osteoporosis (or disuse osteoporosis) observed in patients immobilized for a long period following illness or injuries, but also in astronauts exposed to microgravity [42]. This condition is typically characterized by cortical bone loss, while trabecular bone loss is commonly observed in other osteoporotic conditions, and is the consequence of a reduced mechanical load on bone, a physical stress mediated by the osteocytes, and altered bone remodeling [42]. It also includes drug-induced osteoporosis, a highly prevalent disorder associated with a prolonged drug treatment [43, 44]. Glucocorticoids are one of the best studied examples. They impair osteoblast differentiation by dysregulating the WNT/β-catenin signaling pathway [45], and also stimulate osteoblast apoptosis [46]. Indirectly, glucocorticoids affect osteoblast function by reducing the expression of insulin-like growth factor 1 (IGF-1) [47], which promotes bone formation by mediating the anabolic effects of the parathyroid hormone (PTH) [48]. Glucocorticoids can also stimulate osteoclastogenesis by reducing the production of osteoprotegerin (OPG) by osteocytes and osteoblasts [49], further favoring bone loss. Therapeutic approaches for osteoporosis comprise a set of bone anabolic and antiresorptive therapies, which are used with the main objective of preventing bone loss, increasing bone formation, and reducing the fracture risk. The advantages and disadvantages correlated to each of the major groups of pharmacological agents currently implemented will be discussed in the next section. Importantly, all therapeutics currently approved are characterized by long-term limited efficacy and side effects.
Disorders caused by an impaired osteoclast function
These pathologies are characterized by a vast group of rare, primary monogenic disorders gathered under the name osteopetrosis, also known as the marble bone disease. Osteopetrosis is characterized by a defective bone resorption, increased bone mass and high BMD, and is associated with bone fragility and an increased risk of fractures, and, in some cases, with defective bone marrow, kidney, and nervous and immune systems [50]. There are two prevalent forms of osteopetrosis, which are distinguishable based on their inheritance modality. A more prevalent, milder, and typically late-onset form (arising late during childhood) known as autosomal dominant osteopetrosis (ADO), and a more rare, aggressive and early-onset form (arising early after birth) associated with severe phenotypes and poor prognosis, known as autosomal recessive osteopetrosis (ARO) [50]. ARO can be subdivided into osteoclast-poor and osteoclast-rich forms, depending on whether the mutation at the origin of the disease affects a gene linked to osteoclast differentiation or resorptive function [50]. In addition, a rare form of X-linked osteopetrosis (XLO) has also been described [51]. Mutations in genes that are central to osteoclast function have been associated with the etiology of osteopetrosis, in particular those involved in the acidification of bone microenvironment (TCIRG1, CNCL7), degradation of the extracellular matrix (CTSK), and cell differentiation (RANK, RANKL, CSF1R, NEMO, RELA) [52]. There are currently no pharmaceutics to efficiently treat osteopetrosis, and therapeutic approaches are only aimed at managing symptoms and relieve pain, e.g., supplementation of vitamin D and calcium in patients with hypercalcemic seizures, transfusion of red blood cells and platelets in patients with bone marrow failure, transplantation of hematopoietic stem cells in patient suffering from the most severe forms of osteopetrosis [50].
What is on the menu? Current therapeutic strategies, their efficacy, and limitations
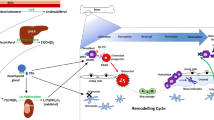
Therapeutic solutions currently available to treat MBDs fail to meet the clinical demand. Drugs lack either efficacy or are only effective for a limited time window, or trigger long-term use-associated side effects, affecting their compatibility with the needs of patients with life-lasting chronic conditions. In the following sections, we will briefly present therapeutics currently in use, their efficacy, and limitations. Figure 1 exemplifies the main groups of bone erosive disorders, therapeutic approaches, and molecular targets.
Molecular mechanisms of bone metabolic disorders (red boxes and arrows) and therapeutic treatments (green boxes and arrows) currently available. A complex network of organs, tissues, and signals intervein to control bone metabolism and a large number of emerging therapeutic targets are being described. Symbols: continuous lines with pointed arrowheads indicate process upregulation; continuous lines with blunt arrowheads indicate process downregulation; dashed lines with pointed arrowheads indicate an intermitted stimulation causing process upregulation. UV, ultraviolet radiation; Ca/PO4, inorganic calcium and phosphate ions; 7-DHC, 7-dehydrocholesterol; VD3, vitamin D3 (also known as cholecalciferol); 25(OH)D3, 25-hydroxyvitamin D3 (also known as calcifediol); 1,25(OH)2D3, 1,25-dihydroxyvitamin D3 (also known as calcitriol); PTH, parathyroid hormone; E2, estradiol; SOST, sclerostin; WNT, canonical Wnt signalling pathway; LRP5/6, low-density lipoprotein receptor-related protein 5/6; RANK, receptor activator of nuclear factor κB; RANKL, RANK ligand; H + , proton; H + -ATPase, vacuolar-type proton-ATPase; TRAP, tartrate-resistant acid phosphatase; MMPs, matrix metalloproteinase protein family members; CTSs, Cathepsins protein family members
Vitamin D and calcium supplementation
The central roles of calcium [53] and VD [54] in bone health are long-time known. Still, there is no consensus on the dose that should be recommended to healthy individuals and patients with increased fracture risk [55, 56], nor whether benefits accompanying the supplementation of calcium and VD outweigh associated risks [57]. Calcium supplementation has little or no effect on the reduction of fracture risk in healthy individuals [58] but can reduce fracture risks and increase BMD in postmenopausal women [59, 60]. It has been associated with an increased risk of cardiovascular disease [61], although this association was refuted in a recent meta-analysis of the clinical data [62]. The source of calcium is certainly an important aspect and several studies reported that natural sources of calcium are more efficient in preventing bone loss than synthetic analogs [63]. VD supplementation, alone or in combination with calcium, has little or no effect on the reduction of fracture risk or increase of BMD in healthy individuals [64, 65] but is associated with a reduced risk of falls in elderly [66] and a reduced bone loss in postmenopausal women [67]. However, several studies highlighted that the supplementation of VD or calcium alone cannot rescue bone loss once it has already occurred [68, 69]. The combination of calcium and VD was also not associated with an increased risk of cardiovascular disease or mortality [62]. Recently, alfacalcidol [1-α-(OH)D3], a vitamin D3 analog, was found to be more effective for the treatment, rather than the prevention, of postmenopausal osteoporosis, glucocorticoid-induced osteoporosis (GIOP), and osteomalacia, when compared to cholecalciferol [70].
In relation to their application to diseases other than osteoporosis, VD and calcium supplementation represent the primary tool for the prevention and treatment of osteomalacia and nutritional rickets, and have demonstrated to be a rapid and effective therapy to restore BMD and serum biomarkers but also to relieve symptoms [71]. However, the restoration of bone density and healing of bone fractures may take time (months) and bone loss may be irreversible at some particular sites [72]. VD and calcium supplementation at low doses is also used in the treatment of primary and secondary hyperparathyroidism [73], to restore plasma levels and prevent the deficiency of both molecules in patients with abnormal PTH production or renal insufficiency. In hyperparathyroidic patients undergoing parathyroidectomy, VD and calcium supplementation is used to prevent post-surgery hypocalcemia [74]. VD supplementation also finds application in the treatment of Paget’s disease of bone, to counteract hypovitaminosis D, which appears to be frequent in pagetic patients [75], but also to prevent flu-like symptoms commonly observed in patients treated with bisphosphonates [76]. Treatment with high doses of calcitriol was tried in ARO patients to stimulate osteoclast differentiation but resulted in poor outcomes [77]. As such, its use is currently not supported by clinicians [78]. Nowadays, calcium and cholecalciferol supplementation is encouraged for osteopetrotic patients to prevent the hypocalcemic seizures that are frequently associated with this condition due to the immobility of calcium from the bone [78].
Vitamin K supplementation
The term vitamin K (VK) collectively refers to a group of fat-soluble compounds found in animals and plants, represented by three main vitamers: phylloquinone (VK1), menaquinones (VK2), and menadione (VK3). The central role of vitamin K in animal physiology has been largely associated with its function as cofactor of the γ-carboxyglutamyl carboxylase (GGCX), a cytosolic enzyme which catalyzes the carboxylation of Glu into Gla residues and the functionalization of the vitamin-K-dependent proteins (VKDPs) [79], which include proteins important for bone matrix organization and mineralization such as the bone Gla protein (BGLAP or osteocalcin), matrix Gla protein (MGP), and Gla-rich protein (GRP or UCMA) [79]. Vitamin K also regulates bone metabolism in a GGCX-independent manner by binding the pregnane X receptor (PXR, SXR or NR1I2), which controls the expression of genes involved in osteoblastogenesis, osteoclastogenesis, and extracellular matrix formation and mineralization, ultimately affecting bone mechanical properties [79]. Because VK plasma levels in healthy individuals are low and detection is rather difficult, little data are available on the pathology and epidemiology of VK deficiency [79]. VK deficiency has been associated with cardiovascular disorders including neonatal bleeding [80], and vascular calcification in patients suffering from CKD [81]. In patients with end-stage CKD, VK deficiency is also associated with bone loss in the osteopenic range and increased fracture risk [82]. Other chronic disorders leading to secondary VK deficiency have also been associated with skeletal comorbidities. For instance, patients suffering from Crohn’s disease have a lower BMD associated with VK deficiency possibly due to intestinal malabsorption [83]. Despite accumulating evidence on the central role of VK in bone health, its supplementation in postmenopausal and osteoporotic patients did not significantly improve BMD and incidence of fractures [84]. Interestingly, some studies suggest that a combined treatment with VK, VD, and calcium may provide a protective effect against bone loss [85, 86].
Supplementation of n-3 polyunsaturated fatty acids (PUFAs)
Polyunsaturated fatty acids (PUFAs) are important regulators of bone metabolism [87]. Fatty acids derivatives, such as eicosanoids and docosanoids are formed upon PUFA oxidation by cyclooxygenases, lipoxygenases, and epoxygenases, and act as anti- and pro-inflammatory molecules, respectively, regulating the equilibrium of bone remodeling [88]. For example, prostaglandin E2, a pro-inflammatory cytokine derived from arachidonic acid, can promote osteoclastogenesis and inhibit osteoblastogenesis [88]. PUFAs can also impact directly on bone cells, with n-3 PUFAs inducing proliferation of bone marrow mesenchymal stem cells while stimulating osteoblast differentiation, and n-6 PUFAs stimulating osteoclastogenesis [88]. PUFA derivates are also natural ligands of the peroxisome proliferator-activated receptor gamma (PPARγ), which is an important molecular switch that deviates the fate of mesenchymal stem cells (MSCs) from osteogenesis towards adipogenesis [88]. Multiple animal studies conducted in ovariectomized (OVX) rats and mice showed that dietary supplementation with n-3 PUFAs decreased osteoclastogenesis [89], reduced bone loss [90], and promoted chondrocyte-to-osteoblast transdifferentiaton [91].
The relative consumption of n-3 and n-6 PUFAs can also regulate the composition of bone cell membranes in fatty acids [92]. In this regard, dietary strategies that reduce n-6/n-3 ratio have been proposed for the treatment of bone erosive disorders. Two recent meta-analyses of randomized controlled trials conducted in human patients confirmed that the supplementation of n-3 PUFAs, with α-linolenic acid (ALA) being more potent than EPA and DHA, was able to slightly increase BMD, reduce resorption markers and, in the case of ALA, slightly increase bone formation markers in a short term. A stronger effect was observed in postmenopausal women [93, 94]. It is worth noting that the positive effects of PUFA supplementation reported in these studies are very low when compared with the effect of pharmaceuticals used to treat osteoporosis.
Extracellular calcium-sensing receptor modulators
Extracellular calcium-sensing receptor (CaSR) is a central regulator of PTH secretion by the parathyroid glands in response to variations of calcium levels in the serum of higher vertebrates, and is, therefore, a key target in drug discovery for disorders characterized by the dysregulation of calcium mineral homeostasis [95]. CaSR activators, also known as calcimimetics, are molecules acting as CaSR agonists or allosteric activators. By binding CaSR, they inhibit PTH secretion and re-equilibrate parathyroid function in patients suffering primary, secondary, and tertiary hyperparathyroidism. Several calcimimetic drugs are used to treat hyperparathyroidism following parathyroid hyperplasia, parathyroid cancer, chronic kidney disease, and kidney transplant [95, 96]. Among those, cinacalcet has been approved for the treatment of patients with secondary and primary hyperparathyroidism that cannot or refuse to undergo parathyroidectomy. Evidences from case studies and randomized controlled trials highlighted the efficacy of cinacalcet in lowering PTH and serum calcium levels, in accordance with results in mammalian models [96]. Cinacalcet also improved bone turnover markers and bone histology but exhibited a poor ability, or none, in increasing BMD [127, 128]. Few calcimimetics are currently being evaluated in drug discovery pipelines, mainly because in vitro high-throughput technologies are missing and screening is limited to whole animal testing [95]. Calcilytics, allosteric antagonists of CaSR stimulating the secretion of PTH by the parathyroid glands, have been proposed to treat patients suffering from primary osteoporosis after several studies reported the osteoanabolic potential of transient PTH exposure [95]. Despite promising results in OVX rats [97], calcilytics did not confirm their potential in human and no reasonable advantage over PTH analogs was found. As a result, clinical trials for most of candidate calcilytics were discontinued [95, 96].
Antiresorptive agents
Antiresorptive drugs inhibit bone resorption either by impairing osteoclast differentiation, recruitment or activity, or by promoting osteoclast apoptosis [98]. Estrogens are potent inhibitors of bone resorption and have been used in hormonal replacement therapy following menopause to increase BMD and reduce fracture risks [31]. Unfortunately, estrogen treatment was associated with an increased risk of breast and uterine cancers and cardiovascular diseases, and has progressively slipped out the list of potential treatments for postmenopausal OP [99]. Selective estrogen receptor modulators (SERMs) are drugs that can specifically modulate the activity of bone specific isoforms of the estrogen receptor; thus, they trigger the beneficial effect of estrogens over bone without increasing the risk of breast and uterine cancer [98]. Two SERMs currently approved for the treatment of postmenopausal OP, raloxifene and bazedoxifene, have demonstrated a mild positive effect on reducing fracture risk [31]. However, they have also been associated with both mild and rare but severe cardiovascular side effects [31]. Testosterone replacement therapy has proven to be effective in increasing BMD in men with osteopenia and osteoporosis [100], although several studies have associated it with an increased risk of cardiovascular diseases [101].
The peptide hormone calcitonin is a potent inhibitor of osteoclast activity [102], and both human and salmon calcitonins have been used as an antiresorptive treatment for OP, PDB, and hypercalcemia, in both injectable and nasal spray forms [103]. However, several studies associated the use of calcitonin with an increased risk of prostate cancer in men, prompting the removal of calcitonin from the list of approved therapies for osteoporosis by the European Medicine Agency (EMA) in 2012 [104]. Nowadays, calcitonin therapy is limited to pagetic patients and short treatments are recommended.
Cathepsin K (CTSK), a cysteine protease primarily involved in the degradation of bone extracellular matrix and produced in large quantities by active osteoclasts, has also been targeted by antiresorptive drugs. CTSK inhibitor odanacatib was assessed in clinical trials [105], and available data indicated a reduction of bone resorption markers and an increase of BMD in a dose-dependent manner [106]. However, positive effects quickly disappeared once the treatment was discontinued [107]. Because odanacatib was also associated with an increased risk of stroke in osteoporotic woman, all trials were discontinued [108].
Bisphosphonates are chemically stable analogs of inorganic pyrophosphate (PPi) with antiresorptive properties. They have been successfully used for nearly 4 decades to treat bone remodeling disorders including postmenopausal OP, age-related and immobility-induced OP, GIOP, PDB, and hyperparathyroidism [16, 98, 109, 110]. Although the implementation of bisphosphonates in clinical practice largely preceded the full understanding of their mechanism of action, an intense research effort during the last 2 decades shed some light over the molecular basis of bisphosphonate action on bone cells. Briefly, bisphosphonates bind to hydroxyapatite crystals at active sites of bone remodeling sites, then are incorporated into osteoclasts following bone resorption, where they inhibit the post-translational modification of proteins involved in cell function, ultimately leading to cell death [111]. Because of their high affinity for calcium, bisphosphonates tend to accumulate in bone, being released by osteoclasts only at active remodeling sites. Therefore, bisphosphonates are typically administered on a weekly, monthly or even yearly basis. Bisphosphonates commonly used to treat bone related disorders—alendronate, risedronate, ibandronate, and zoledronate—are able to decrease bone resorption up to 70% and reduce the incidence of vertebral and non-vertebral fractures in women with osteoporosis up to 62% and 40%, respectively [130].
Denosumab is a RANKL monoclonal antibody approved for the treatment of postmenopausal OP, age-related OP, and GIOP [112], but also PDB, primary and secondary hyperparathyroidism. Denosumab binds to RANKL with a high affinity, mimicking the activity of the endogenous OPG and preventing its ligation to RANK receptor at the osteoclast surface, therefore inhibiting the major signaling cascade involved in osteoclast differentiation [113]. Denosumab is a potent inhibitor of bone resorption that can reduce the incidence of vertebral, non-vertebral, and hip fracture in osteoporotic patients of 68%, 20%, and 40%, respectively, thus has an efficacy similar to that of bisphosphonates and osteoanabolic drugs [113]. As for other antiresorptive agents, patients treated with Denosumab experience a steep increase in BMD in the first 6–12 months after the beginning of the treatment, but while bisphosphonate treatment has been associated with a steady BMD after this first period, Denosumab produces a slow but continuous increase in mineral density [114]. Denosumab has also shown some efficacy in rescuing bone remodeling markers in both old and juvenile pagetic patients [115, 116], and in patients with hyperparathyroidism [117].
Bisphosphonates and Denosumab have been correlated to mild and frequent but also rare and severe side effects, raising concerns among clinicians. Among those more severe but rare, atypical femur fracture was reported in 1 patient out of 250 (frequency increases with the duration of the treatment), and osteonecrosis of the jaw was observed in 1 patient every 4000 [118]. Among those less severe but frequent upper gastrointestinal side effects, increased risk of esophageal cancer (still uncertain), musculoskeletal pain and flu-like symptoms were reported for bisphosphonates [115]. Denosumab may reduce bone turnover, a secondary effect that should be considered when treating CKD patients because of the risk of facilitating the development of adynamic bone disease [115]. Serum levels of calcium and VD must be monitored before and during Denosumab treatment due to increased susceptibility to hypocalcemia [115]. Furthermore, Denosumab treatment has been associated to increased risk of adverse effects to infections, presumably due to its immunosuppressive properties [119].
Despite their positive effect, last-generation antiresorptive drugs are characterized by a limited long-term efficacy. Indeed, although they can prevent further loss of mineral, they do not rescue the irreversible deficit in bone volume that occurs in metabolic bone disorders [114]. Several authors have proposed that the increase in BMD observed following the treatment with antiresorptive agents may only be an artefact resulting from the secondary mineralization of already-existing mineral matrix, and may not be associated with the deposition of new ECM and increase in bone volume, which are needed for structural improvement and protection against fragility fractures [114]. Furthermore, a discontinuation of antiresorptive therapy is typically associated with a re-increase in bone resorption and subsequent mineral loss [120]. As such, clinicians and researchers are currently evaluating the co-application or the sequential application of antiresorptive and osteoanabolic agents (see below).
Osteoanabolic agents
Osteoanabolic drugs have the capacity to impact on the formation and mineralization of the extracellular matrix orchestrated by osteoblasts. It is increasingly admitted that only an osteoanabolic approach can ultimately compensate for the loss of bone volume observed in low-BMD disorders [114]. Yet, there is a surprising scarcity of bone anabolic compounds available to patients.
Among the few drugs used to restore bone mineral density, strontium ranelate was long considered the most promising osteoanabolic compound after several studies reported increased BMD and reduced fracture risk in treated patients [121]. However, its association to increased cardiovascular events and myocardial infarction in postmenopausal women led to the discontinuation of its production [122], and nowadays its use is not approved any longer by the European Medicine Agency. Two other osteoanabolic drugs are available for osteoporotic patients in Europe: teriparatide, the synthetic analog of the peptidic parathyroid hormone (PTH), and abaloparatide, the analogue of the parathyroid hormone-related peptide (PTHrP). The dualistic action of PTH on bone metabolism and the anabolic effect of an intermittent treatment with PTH—rather than the classical catabolic effect associated with the continuous exposure to PTH—is known for a long time [123]. Early studies identified osteoblastic lineage as the primary target for PTH regulation of bone homeostasis [124] and that exposure to low dosage of PTH for short periods indeed triggers the proliferation of osteoblast precursors [125]. Subsequent studies revealed that PTH stimulates osteoblast differentiation by stimulating pro-osteogenic WNT signaling pathway and inhibiting pro-adipogenic PPARγ signaling pathway in MSCs [126, 127]. PTH also inhibits apoptosis in osteoblastic cells, contributing to more cells being available for bone formation and mineralization [128]. The pro-resorptive effect of constantly elevated serum levels of PTH (e.g., during the development of hyperparathyroidism) was attributed to the stage-specific capacity of PTH to induce the expression of RANKL and inhibit OPG expression throughout osteoblast differentiation [129]. The PTH synthetic analogue teriparatide (hPTH 1–34) is composed of PTH bioactive region (amino acids 1 to 34). It is currently approved worldwide for the treatment of postmenopausal OP, age-related OP, and GIOP, and can reduce up to 80% of vertebral fracture and 50% of non-vertebral fractures in osteoporotic patients, representing one of the most effective treatment currently available [114, 130]. Teriparatide can also alleviate bone phenotypes associated with genetic disorders such as osteogenesis imperfecta [131]. Despite an excellent short-term efficacy, the long-term use of teriparatide has faced several limitations, e.g., the necessity of parenteral administration (which affect the patient’s compliance with the treatment due to side effects related to repetitive injections), and secondary effects such as decreased BMD in the radius, dizziness, leg cramps, headache and hypercalcemia [130]. Due to the dualistic effect of PTH on bone and a short-term efficacy, teriparatide will trigger an osteoanabolic effect for 12–24 months (period known as the anabolic window), then a catabolic effect characterized by increased osteoclast activity and bone resorption. Unfortunately, bone loss will occur even if treatment is discontinued [114, 132]; thus, teriparatide treatment is frequently followed by an antiresorptive therapy [114, 132].
When compared to PTH, PTHrP triggers a similar osteoanabolic action but has a milder pro-resorptive effect and a lower tendency to induce hypercalcemia. This could be related to the different affinity of PTH and PTHrP for different conformational status of the receptor PTHR1, influencing the receptor kinetic with consequence a milder stimulation of the downstream signaling cascade [130]. Based on the superior performances of PTHrP, the synthetic analog abaloparatide (PTHrP 1–34) was recently developed. It is not yet approved for the treatment of osteoporotic patients in Europe but several studies have highlighted the similar effect of teriparatide and abaloparatide in increasing BMD, and a very similar or higher effect in preventing vertebral and non-vertebral osteoporotic fractures [130]. Abaloparatide was also claimed to have a better anabolic window than teriparatide due to a lower pro-resorptive effect over time [132]. However, this claim is only supported by clinical evidence of a delayed increase in serum resorption marker C-terminal telopeptide of type 1 collagen (CTX) following Abaloparatide treatment and challenged in several studies [114]. It is worth to mention that the administration of teriparatide and abaloparatide to patients with a high risk of cancer, e.g., pagetic patients, is discouraged in the USA as it may favor the development of osteosarcoma, a warning based on studies performed in rats [133]. Yet, in 35 years of approved clinical use of teriparatide (abaloparatide was only approved in 2017), no concrete evidence of an increased incidence of osteosarcoma in humans was reported [134].
Co-administration and sequential administration of osteoanabolic and antiresorptive drugs
Because monotherapies have shown some limitations, the efficacy of combinational therapies—i.e., the co-administration or sequential administration of antiresorptive drugs and osteoanabolic agents—has been evaluated, reviewed in [135], and results are contrasted. The co-administration of bisphosphonates and Denosumab did not clearly improve outcomes of monotreatments [98], while the combination bisphosphonate and estrogen only resulted in a slightly better BMD [135]. A recent meta-analysis of randomized controlled trials indicated that patients co-treated with teriparatide and antiresorptive agents showed an improved BMD gain and a reduced risk of fracture [136]. Sequential treatment with antiresorptive agents was only beneficial if the second treatment was done with a more potent antiresorptive; in that case, effect of the first treatment could be maintained [135]. Sequential treatments with different types of drugs have proven to be more effective. Consequently, a treatment with bisphosphonates or Denosumab following an initial treatment with bone anabolic drug could prevent bone loss commonly observed after monotherapies of osteoanabolic agents, and maintain or further increase gains in BMD [98]. However, this ideal setup has not been applied yet in clinics, where most patients are typically treated first with an antiresorptive drug, then with another antiresorptive drug or an osteoanabolic agent, whenever fracture risk is consistently high. Available evidence shows that the positive effect of teriparatide is higher in naïve patients (that never received an antiresorptive agent before) than in those receiving the treatment following an antiresorptive therapy, suggesting that the reduced rate of bone remodeling induced by antiresorptive may be blunting the remodeling-based gain in BMD triggered by osteoanabolic drugs [114]. However, the substitution of an antiresorptive therapy by an anabolic therapy appears to be overall beneficial to patients, at least regarding gain and maintenance of BMD, although the effect of this therapeutic sequence on fracture risk has yet to be evaluated [135].
Dual-action agents
Romosumab is a human monoclonal anti-sclerostin antibody, whose use was approved in USA and EU in 2019 for osteoporotic patients presenting a high risk of fracture. Sclerostin is produced by osteocytes and serves as a master regulator of bone formation through its binding to LRP5/6 receptors and the subsequent inhibition of WNT/β-catenin canonical signaling pathway, which is paramount for osteoblast differentiation and metabolism [137]. Romosumab also increases OPG expression and consequently inhibits osteoclast differentiation [132]. Therefore, Romosumab action on sclerostin promotes bone anabolic and antiresorptive effects, which is the rationale for considering Romosumab as a dual-action drug. Clinical trials have demonstrated that Romosumab treatment induces a rapid increase in bone formation markers, an increase in BMD and an equally rapid decrease in bone remodeling markers [132]. A number of randomized controlled trials have highlighted the capacity of Romosumab to reduce the incidence of fragility fractures to an extent comparable, if not superior, to the effect of bisphosphonates and teriparatide [114, 132]. Romosumab is characterized by a short and powerful anabolic window that triggers a rapid increase in bone formation during the first months of treatment. However, after few months, Romosumab anabolic window dissipate and is substituted by a mild antiresorptive mechanism [114, 132]. As such, Romosumab treatment, similar to single-action osteoanabolic drugs, needs to be followed by the treatment with antiresorptive agents [138]. Common adverse effects of Romosumab include headache, arthralgia, and immune reactions at the injection site. An increased risk of cardiovascular events such as myocardial infarction, stroke, and cardiovascular death have been associated with Romosumab treatment [138]. Little is known about Romosumab long-term associated side effects.
Emerging therapeutic approaches for bone disorders
Our knowledge on the molecular determinants of bone metabolism has greatly improved during the last decades, widening the spectrum of potential druggable targets to treat MBDs. Among the molecular regulators recently identified for the treatment of bone-eroding diseases, antiresorptive agents such as H+-ATPase suppressors and Src proto-oncogene inhibitors are promising candidates, as important factors involved in osteoclastic function [139]. Novel potential targets for osteoanabolic agents include intermediates of the WNT/β-catenin pathway such as DKK-1, GSK-3, and Sirt1, activators of the soluble guanylate cyclase (sGC), and bone morphogenetic proteins (BMPs). Hydrogen sulfide donors (H2S), kynurenine pathway blockers, and modulators of the osteoblast–osteoclast crosstalk (e.g., compounds impacting RANKL signaling, cell–cell interaction proteins such as Semaphorins Sema3a and Sema4D, and sphingosine-1-phosphate) are also promising candidates for the development of next-generation dual-action drugs [139].
The identification of crosstalk in cellular signaling pathways central to bone and other tissues and organs has opened the possibility to implement therapeutic strategies with a more holistic approach. Therefore, drugs targeting muscle, fat, and blood vessels are gaining momentum in the treatment of MBDs. For example, activin receptor regulators, a key component of the extracellular matrix involved in osteoclastic differentiation is being studied in animal models [139]. Myokines, factors produced by skeletal muscles, are being described for having a control over bone metabolism and might represent druggable targets for MBDs [139]. Since adipocytes and osteoblasts have a common origin, drugs able to shift the equilibrium from adipogenesis to osteogenesis in MSCs, such as TGFβ- and PPARγ-modulators, are also being evaluated [139]. Similarly, the existence of a crosstalk between endothelium and bone has shed some light on the possibility for angiogenesis regulators to be targeted by therapeutically approaches for MBDs. Among those, intermediates of the Notch signaling pathway and regulators of bone vascularization such as SLIT3 and SHN3 are being evaluated [139]. A crosstalk between gut microbiome and bone health have been identified and the capacity of probiotics and prebiotics to promote bone health has been evidenced [139, 140]. Gut microbiome has also been linked to drug efficacy [141]. Because oxidative stress and inflammation are important factors in the development of MBDs, antioxidant and anti-inflammatory compounds are increasingly being evaluated for their positive impact on bone health [142, 143]. Finally, the interaction between bone and immune system suggests that immunostimulants may also have a beneficial effect on bone [144].
Nowadays, recent advancements in the fields of molecular biotechnologies such as gene therapy, gene silencing, and regenerative medicine have led to the development of innovative biotechnological approaches for treating metabolic bone disorders. Among those, a recombinant RANKL-based vaccine has shown to be able to prevent osteoporosis in OVX mice [145]. An adenovirus-delivered microRNA-based gene silencing method was able to prevent bone loss in a mice osteoporotic model by silencing RANK and CTSK expression [146]. In addition, a gene delivery system that enhances the specific bone delivery and distribution of miRNA was also developed [147]. Stem cell transplantation technologies can also be applied to the treatment of metabolic bone disorders. In this regard, the transplantation of MSCs has shown promising results in pre-clinical studies, and clinical trials are currently being conducted in osteoporotic patients [148]. MSCs-derived extracellular vehicles (EVs) have also drawn some attention because of their osteogenic potential [149]. Hematopoietic stem cells transplantation, a well-established life-saving therapeutic option for malignant infantile osteopetrosis [150], has been recently applied to the treatment of patients suffering from the less-severe autosomal dominant form of osteopetrosis [151, 152]. A combinational strategy based on the transplantation of autologous hematopoietic stem cells where the disease-causing mutation was previously corrected through gene therapy delivered via lentivirus transformation has been adopted with success in an osteopetrotic mice model [153].
Marine natural products as alternative players in MBD therapeutic strategies
Historically, natural products (NPs) have played a central role in the advancement of pharmacology, and they are still today the basis of many contemporary pharmaceutics. Although their use in pharmaceutical research has slowed down in the early 1990s due to technical limitations related to a poor compatibility with high-throughput screening approaches, recent biotechnological advances and the advent of the “omic” sciences have placed them back in screening pipelines for novel drugs [154, 155]. In addition, the diversity of the bioactivities found in NPs, but also their chemical novelty, and effectiveness in leading to the discovery of first-in-class medications (i.e., drugs that perform through novel and unique mechanisms of action), are features that have contributed to their leading role in drug discovery. As such, only 24.6% of all drugs approved by FDA in the last 4 decades were purely synthetic, while the remaining were either fully natural (4.6%), naturally derived (18.9%), biological (isolated from an organism/cell line or produced in a surrogate host; 18.4%), biologically produced vaccines (7.5%), natural product mimics or synthetic compounds whose bioactive portion is naturally derived (25.7%) [156]. In this new era of NP-inspired drugs, the marine environment is increasingly seen as a valuable reservoir of bioactives because of its vast yet largely unexplored biodiversity in contrast to the much more explored terrestrial environment [157].
Animals as first-choice resources in marine pharmacology
Terrestrial plants (25%) and microorganisms (13%) are traditionally the main contributing organisms for bioactives used in disease management, in particular for bone erosive disorders [158,159,160]. However, animals are the primary source of compounds from the marine environment. A comprehensive review on this topic has estimated that approximately 75% of the marine compounds were isolated from invertebrates, the major phyla being Porifera (marine sponges) with 32%, and Cnidaria (e.g., corals, jellyfishes, anemones, and sea fans) with 16%. Other important groups such as Mollusca (mollusks) contributed with 5%, Echinodermata (e.g., starfish, sea urchins, and sea cucumbers) with 5%, and Chordata (e.g., tunicates and vertebrates) with 4% [161]. Despite a large untapped biodiversity, marine microorganisms contributed 22–34% of the total bioactive compounds discovered in the marine environment [161].
Marine osteoactive compounds (MOCs)
Compounds isolated from marine organisms hold a great potential for the treatment of MBDs [162]. Still, limited research effort has been put on the discovery of marine compounds with osteoactive properties. This section will review the literature data on the isolation of marine osteoactive compounds from 1999 to 2023. Note that only compounds with pharmacological applications will be presented here; marine-derived biomaterials with applications in bone regeneration, fracture healing, and tissue engineering will be overlooked since it has already been reviewed [163,164,165,166]. Our survey identified a total of 101 marine osteoactive compounds (Fig. 2B), of which 54 (53.5%) are antiresorptive, 34 (33.7%) are osteoanabolic, 12 (11.9%) have a dual-action, and 1 (0.9%) is anti-osteonecrotic (Table 1). Our survey also revealed an overall scarcity of studies, with only 90 papers published between 1999 and 2023 about the isolation of new MOCs. However, the last 2 decades have seen a steadily increase in these studies (Fig. 2A), which is in agreement with the overall increment of all-type marine bioactives reported previously [157]. As such, a significant increase in the research effort aiming at the discovery of osteoactive compounds from marine organisms is anticipated in the upcoming years. The taxonomic distribution of the organisms contributing to MOCs is shown in Fig. 3. Animals (46 compounds, mostly from invertebrates) are the largest contributors (Fig. 3A), followed by algae (22, mostly from large pluricellular brown algae), fungi (20, all from ascomycetes), and bacteria (14, mostly from cyanobacteria). The distribution of MOCs at Phylum level (Fig. 3B) revealed that fungi (Ascomycota) and sponges (Porifera) provided the highest number of MOCs (20% and 17%, respectively), followed by brown macroalgae (Ochrophyta, 14.9%), corals (Cnidaria, 12.9%), cyanobacteria (10.1%), Chordata (6.9%) and Mollusca (5.9%). Dinoflagellates (Dinoflagellata), green- and red algae (Chlorophyta and Rhodophyta), crustaceans (Arthropoda) and worms (Anellida) collectively accounted for the remaining MOCs (4%). This data, although limited to a reduced set of compounds, validates the suitability of marine organisms as sources of natural bioactives for marine pharmacology.
Interestingly, MOC distribution resembles the tendency previously described for all-type marine bioactives [161], an indication that a similar sampling effort was directed toward these groups. Also of interest, ten of the fungi-related MOCs were isolated from species that live in close symbiotic relationships with marine sponges (5), corals (3), seaweeds (1), and mangroves (1).
Future perspectives
Underexplored groups as promising sources of MOCs
Many groups of marine organisms are underrepresented in the current screening scenario. Among those, marine algae have provided a plethora of bioactive compounds [254], and several studies support the idea that they represent a promising source of pharmacologically relevant osteoactive compounds. In this regard, mineral-rich extracts prepared from the red coralline algae Lithothamnion spp. have pro-mineralogenic properties that partly rescue bone loss in osteoporotic animal models [255]. Extracts prepared from green (Codium fragile and Cladophora rupestris) [303 and red (Plocamium cartilagineum and Ceramium secundatum) [256] macroalgae also showed pro-mineralogenic activity in fish osteochondroprogenitor cells and pro-osteogenic activity in zebrafish. Red (Dichotomaria obtusata) and brown (Padina pavonica) macroalgae triggered pro-osteoblastogenic signals in mouse bone marrow MSCs [257] and human primary osteoblasts [258]. Recently, calcium-chelating peptides derived from several species of marine microalgae could rescue osteoporotic phenotypes in zebrafish [259]. It is worth mentioning that the large-scale production of algal biomass is supported by a well-established and technologically advanced industry. Of special interest, microalgae have been long cultivated for nutritional, biotechnological, and industrial applications and are being used for the production of food, dietary supplements, cosmetics, pharmaceuticals, biofuel, fertilizers, but also for wastewater treatment [260]. Following important biotechnological advancements that improved growth conditions and allowed the establishment of genetically modified strains optimized for growth and compound biosynthesis [261], microalgae are expected to become highly relevant species for marine pharmacology in the upcoming years. In this regard, ethanolic extracts prepared from two species of microalgae (Skeletonema costatum and Tetraselmis striata) were recently shown to contain potent osteoactive compounds [262].
Marine invertebrates such as mollusks, gastropods, and echinoderms are also promising sources of osteoactive compounds. Among the mollusks, bivalves such as mussels, oysters, clams, and scallops have originated peptides, polysaccharides, and glycoproteins with antioxidant and anti-inflammatory activity, and lipids and polyunsaturated fatty acids with strong anti-inflammatory and anti-arthritic properties [263]. Osteoanabolic [250] and antiresorptive compounds have also been isolated from bivalves. Among those, the nacre, also known as mother of pearl, has both osteoinductive and antiresorptive properties [264, 265]. Fermented extracts of the oyster Crassostrea gigas have also a dual-action activity, stimulating osteogenic differentiation via Wnt and IGF pathways [266, 267] and suppressing osteoclast differentiation, thus preventing OVX-induced bone loss in mouse [268]. Similarly, aqueous extracts of the bivalve Pisidium coreanum showed anti-osteoclastogenic activity and were able to rescue osteoporosis in OVX mice [269]. Among the gastropods, methanolic extracts of the brown dwarf turban (Turbo brunneus) and the sea snail Euchelus asper prevented bone loss [270] and improved osteoporotic phenotype [271], respectively, in OVX mice. Echinoderms such as sea urchins, starfish and sea cucumbers are at the origin of about 5% of all the marine bioactives discovered so far [161]. In the context of this review, polyhydroxylated naphthoquinones extracted from the sea urchin Evechinus chloroticus increased ECM mineralization in human osteosarcoma cells when administered together with calcium chloride, but decreased it when administered alone [272]. Sea cucumbers also hold a great deal of potential with both osteoanabolic [273] and antiresorptive [274] extracts identified.
Among chordates, ascidians such as sea squirts are well-known sources of compounds with anticancer, antimicrobial, and antioxidant activities, some of which are being currently evaluated in clinical trials [275]. Compounds with osteoactive properties have also been isolated from ascidians [199, 236], and extracts with antioxidant and anti-inflammatory activities have recently been found to also exhibit pro-osteogenic properties [276]. In vertebrates, bone-derived gelatin from the saffron cod (Eleginus gracilis) and skin-derived gelatin from the blue shark (Prionace glauca) have shown protective properties against bone loss in OVX rats [277, 278], while bone powder from tuna (Thunnus spp.) could reduce bone loss in a GIOP mice through the co-regulation of NF-κB and Wnt/β-catenin pathways and the modulation of gut microbiota composition and metabolism [279].
Finally, dichloromethane and ethanolic extracts of halophyte plants Salicornia herbacea and Spergularia marina, respectively, were reported to have anti-adipogenic and pro-osteoblastogenic activities in vitro [280, 281]. Recently, polyphenols-rich extracts of Spartina alterniflora and Salicornia fragilis were found to have pro-mineralogenic activity in fish osteochondroprogenitor cells and pro-osteogenic activity in zebrafish [282].
The availability of animal models and screening tools is not fully exploited
The global interest for underexplored marine organisms as a source of osteoactive compounds has steadily increased in the last 2 decades following the demonstration that they produce osteoanabolic and antiresorptive compounds. However, the discovery of novel MOCs is only achievable through a coordinated effort that should aim at the fractionation of the extracts, isolation, and identification of the osteoactive compounds, together with the validation of their biological activity and the elucidation of their mechanisms of action. In this aspect, animal models are increasingly available for compound validation, although only 28% of the compounds listed here were validated in an animal model of metabolic bone disorders (Fig. 4), while the vast majority, i.e., 72%, were only tested in vitro, mainly using rodent cell lines. Of the compounds that were validated using in vivo disease models, 25 were tested in animal models of osteoporosis, 3 were tested in mouse models of arthritis, and 1 was tested in a model of bisphosphonate-related osteonecrosis of the jaw. None were tested in animal models of VD-deficiency, hyperparathyroidism, Paget’s disease of bone, or osteopetrosis. Of the compounds tested in animal models of osteoporosis, 18 were tested in rodent models of ovariectomy-induced osteoporosis, 4 were tested in mouse models of LPS-induced bone loss, 1 in a mouse model of D-galactose-induced osteoporosis, and 2 in a zebrafish model of glucocorticoid-induced osteoporosis. In this context, rodents and in particular the mouse, are the preferred animal models in biomedical research due to their genetic similarity with humans, small size, short lifespan, and relatively low maintenance cost compared to other mammalian models [283]. A large variety of mouse models mimicking skeletal disorders are available. The ovariectomized rat and mouse, aim at resembling mechanistically the pathophysiology of postmenopausal osteoporosis and are considered gold-standard in vivo models to validate the efficacy of compounds and drugs with anti-osteoporotic potential [284]. Mouse models that resemble age-related osteoporosis [285], male senile osteoporosis [286], and GIOP [287] are also available to researchers but none of these models have yet been implemented to evaluate the efficacy of MOCs. A rat model of bisphosphonate-related osteonecrosis of the jaw [288] has been successfully used to validate the anti-necrotic potential of a salmon sperm-derived polydeoxyribonucleotide [253]. Great achievements have also been obtained in the modeling of disorders of mineral homeostasis, including vitamin D deficiency [289], primary hyperparathyroidism [290], and renal osteodystrophy [291] using rodents. Models have also been developed for bone genetic disorders such as PDB [292] and osteopetrosis [293].
However, rodent models have technical disadvantages that limit the throughput of screening pipelines for drug discovery. When compared to fish and invertebrate models, rodent systems bring the complexity and the genetic proximity that better resemble humans but are also expensive and more time-consuming. As such, they may be better suited for secondary screenings that aim at validating compound osteoactivity, rather than for primary screenings that mostly serve at funneling down the number of compounds. Teleost fish, in particular the zebrafish (Danio rerio) and the Japanese medaka (Oryzias latipes), are becoming extremely relevant in bone research and can model many human skeletal diseases [294, 295]. These small teleosts offer several technical advantages that make them well suited for drug screening, e.g., smaller size, cost-effectiveness, shorter life span, and higher fecundity when compared to mammalian models. Moreover, the translucency of embryonic stages throughout development and the amenability to gene editing has enabled the generation of a vast array of transgenic and mutant lines that can be used for in vivo-cell tracking and disease modeling [296]. Furthermore, teleost ability to regenerate bone and cartilage tissues offer a different approach for evaluating the osteoactivity of drugs and compounds [297]. As such, a large numbers of drug screening tools have been developed in the latest years based on teleost fish [298, 298], offering a cost-effective, medium- and high-throughput alternative to mammalian-based systems and at the same time providing a level of biological complexity which cannot be yet achieved by in vitro systems. Importantly, several zebrafish and medaka models of human bone disorders are available, including osteoporosis [299], osteopetrosis [300], and PDB [301]. However, teleost models such as zebrafish pose various challenges, including the higher evolutionary distance with humans compared to classical mammalian models, that oftentimes reflects into physiological and anatomical differences [302]. Though, the great advantages offered by these animal models make them very efficient intermediate points between exploratory screening and functional validation of novel osteoactive compounds. Owning to this variety of animal models, it is expected that, in the coming years, the research community working in the field of marine osteoactive compounds will fill the gap in terms of in vivo validation of MOCs.
Conclusion
Metabolic bone disorders and fragility fractures are major causes of reduced welfare, suffering, and morbidity, as well as a tremendous sink of resources for the global health systems. Because most of the drugs currently available are associated with undesirable side effects, there is an unmet demand for effective medications to address metabolic bone disorders. Oceans are increasingly contributing to pharmaceutical research and drug discovery and may hold the solutions to resolve this pressing issue through the production of novel and innovative osteoactive compounds by marine organisms. Our survey of the literature on marine osteoactive compounds identified 101 compounds with antiresorptive, osteoanabolic, or anti-osteonecrotic activities, including compounds with dual activity. It also revealed that marine invertebrates, such as sponges and cnidarians, and microorganisms, such as fungi and cyanobacteria, are major contributors of MOCs, and that future research efforts should explore the untapped biodiversity of marine organisms, such as microalgae, mollusks, holothurians, ascidians, and fishes. To achieve these goals, a cooperative effort between the chemical characterization of marine-derived compounds and the exploitation of drug screening and validation tools currently available will be necessary.
Data availability
All datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wu A-M et al (2021) Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev 2:e580–e592
Feng X, McDonald JM (2011) Disorders of bone remodeling. Annu Rev Pathol Mech Dis 6:121–145
Haseltine KN, Chukir T, Smith PJ, Jacob JT, Bilezikian JP, Farooki A (2021) Bone mineral density: Clinical relevance and quantitative assessment. J Nucl Med 62:446–454
Williamson S et al (2017) Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos Int 28:2791–2800
Saghafi M, Azarian A, Hashemzadeh K, Sahebari M, Rezaieyazdi Z (2013) Bone densitometry in patients with osteomalacia: Is it valuable? Clin Cases Miner Bone Metab 10:180–182
Thacher TD, Fischer PR, Pettifor JM (2014) The effect of nutritional rickets on bone mineral density. J Clin Endocrinol Metab 99:4174–4180
Sheu A, Diamond T (2016) Bone mineral density: Testing for osteoporosis. Aust Prescr 39:35–39
Arruda M et al (2016) Bone mineral density and microarchitecture in patients with autosomal dominant osteopetrosis: A report of two cases. J Bone Miner Res 31:657–662
Tripto-Shkolnik L, Liel Y (2021) Paget’s disease on bone mineral density examination. QJM Int J Med 114:60–61
Koumakis E et al (2014) Individual site-specific bone mineral density gain in normocalcemic primary hyperparathyroidism. Osteoporos Int 25:1963–1968
Malluche HH, Porter DS, Monier-Faugere M-C, Mawad H, Pienkowski D (2012) Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol JASN 23:525–532
Prentice A (2008) Vitamin D deficiency: A global perspective. Nutr Rev 66:S153–S164
Wein MN, Kronenberg HM (2018) Regulation of bone remodeling by parathyroid hormone. Cold Spring Harb Perspect Med 8:a031237
Bhan A, Rao AD, Rao DS (2010) Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin 39:321–331
Elder CJ, Bishop NJ (2014) Rickets. The Lancet 383:1665–1676
Walker MD, Silverberg SJ (2018) Primary hyperparathyroidism. Nat Rev Endocrinol 14:115–125
Mosekilde L (2008) Primary hyperparathyroidism and the skeleton. Clin Endocrinol (Oxf) 69:1–19
Coen G (2005) Adynamic bone disease: An update and overview. J Nephrol 18:117–122
Slatopolsky E, Gonzalez E, Martin K (2003) Pathogenesis and treatment of renal osteodystrophy. Blood Purif 21:318–326
Salari N et al (2021) The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg 16:609
Van Staa TP et al (2002) Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res 17:465–471
Appelman-Dijkstra NM, Papapoulos SE (2018) Paget’s disease of bone. Best Pract Res Clin Endocrinol Metab 32:657–668
Gennari L, Rendina D, Falchetti A, Merlotti D (2019) Paget’s disease of bone. Calcif Tissue Int 104:483–500
Rabjohns EM et al (2021) Paget’s disease of bone: Osteoimmunology and osteoclast pathology. Curr Allergy Asthma Rep 21:23
Meunier PJ, Coindre JM, Edouard CM, Arlot ME (1980) Bone histomorphometry in Paget’s disease: Quantitative and dynamic analysis of pagetic and nonpagetic bone tissue. Arthritis Rheum 23:1095–1103
Gehron Robey P, Bianco P (1999) The role of osteogenic cells in the pathophysiology of Paget’s disease. J Bone Miner Res 14:9–16
Singer FR (2015) Paget’s disease of bone-Genetic and environmental factors. Nat Rev Endocrinol 11:662–671
Malluche HH, Davenport DL, Lima F, Monier-Faugere M-C (2022) Prevalence of low bone formation in untreated patients with osteoporosis. PLoS One 17:e0271555
Yang Y-H et al (2013) Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER–ERK–mTOR pathway. Apoptosis 18:1363–1375
Mann V, Huber C, Kogianni G, Collins F, Noble B (2007) The antioxidant effect of estrogen and selective estrogen receptor modulators in the inhibition of osteocyte apoptosis in vitro. Bone 40:674–684
Shevde NK, Bendixen AC, Dienger KM, Pike JW (2000) Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci 97:7829–7834
Luo CY, Wang L, Sun C, Li DJ (2011) Estrogen enhances the functions of CD4+CD25+Foxp3+ regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol 8:50–58
Krum SA et al (2008) Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27:535–545
Rijhsinghani AG, Thompson K, Bhatia SK, Waldschmidt TJ (1996) Estrogen blocks early T cell development in the thymus. Am J Reprod Immunol 36:269–277
Wu D et al (2021) T-cell mediated inflammation in postmenopausal osteoporosis. Front Immunol 12:687551
Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest 116:1186–1194
Warming L, Hassager C, Christiansen C (2002) Changes in bone mineral density with age in men and women: A longitudinal study. Osteoporos Int 13:105–112
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300
Agidigbi TS, Kim C (2019) Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci 20:3576
Zhang B, Xie Q, Quan Y, Pan X, Liao D (2015) Reactive oxygen species induce cell death via Akt signaling in rat osteoblast-like cell line ROS 17/28. Toxicol Ind Health 31:1236–1242
Porter JL, Varacallo M (2023) Osteoporosis. in StatPearls (StatPearls Publishing)
Rolvien T, Amling M (2022) Disuse osteoporosis: Clinical and mechanistic insights. Calcif Tissue Int 110:592–604
Panday K, Gona A, Humphrey MB (2014) Medication-induced osteoporosis: Screening and treatment strategies. Ther Adv Musculoskelet Dis 6:185–202
Wang L-T, Chen L-R, Chen K-H (2023) Hormone-related and drug-induced osteoporosis: A cellular and molecular overview. Int J Mol Sci 24:5814
Chotiyarnwong P, McCloskey EV (2020) Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol 16:437–447
Zhang S, Liu Y, Liang Q (2018) Low-dose dexamethasone affects osteoblast viability by inducing autophagy via intracellular ROS. Mol Med Rep 17:4307–4316
Delany AM, Durant D, Canalis E (2001) Glucocorticoid suppression of IGF-I transcription in osteoblasts. Mol Endocrinol 15:1781–1789
Canalis E, Centrella M, Burch W, McCarthy TL (1989) Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 83:60–65
Piemontese M, Xiong J, Fujiwara Y, Thostenson JD, O’Brien CA (2016) Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am J Physiol-Endocrinol Metab 311:E587–E593
Stark Z, Savarirayan R (2009) Osteopetrosis. Orphanet J Rare Dis 4:5
Alkhayal Z, Shinwari Z, Gaafar A, Alaiya A (2023) Carbonic anhydrase II activators in osteopetrosis treatment: A review. Curr Issues Mol Biol 45:1373–1386
Palagano E, Menale C, Sobacchi C, Villa A (2018) Genetics of osteopetrosis. Curr Osteoporos Rep 16:13–25
Copp DH (1957) Calcium and phosphorus metabolism. Am J Med 22:275–285
McCollum EV, Simmonds N, Becker JE, Shipley PG (1922) Studies on experimental rickets: xxi. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem 53:293–312
Moyer VA (2013) Vitamin D and calcium supplementation to prevent fractures in adults: U.S. preventive services task force recommendation statement. Ann Intern Med 158:691–696
Zhao J-G, Zeng X-T, Wang J, Liu L (2017) Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: A systematic review and meta-analysis. JAMA 318:2466–2482
Reid IR, Bolland MJ (2019) Controversies in medicine: The role of calcium and vitamin D supplements in adults. Med J Aust 211:468–473
Tai V, Leung W, Grey A, Reid IR, Bolland MJ (2015) Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 351:h4183
Shea B et al (2002) VII Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev 23:552–559
Prince RL et al (2006) Effects of calcium supplementation on clinical fracture and bone structure: Results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med 166:869–875
Myung S-K, Kim H-B, Lee Y-J, Choi Y-J, Oh S-W (2021) Calcium supplements and risk of cardiovascular disease: A meta-analysis of clinical trials. Nutrients 13:368
Zhang Y et al (2021) Association of vitamin D or calcium supplementation with cardiovascular outcomes and mortality: A meta-analysis with trial sequential analysis. J Nutr Health Aging 25:263–270
Omelka R et al (2021) Chicken eggshell powder more effectively alleviates bone loss comparted to inorganic calcium carbonate: An animal study performed on ovariectomized rats. J Physiol Pharmacol 72:873–879
Winzenberg T, Powell S, Shaw KA, Jones G (2011) Effects of vitamin D supplementation on bone density in healthy children: Systematic review and meta-analysis. BMJ 342:c7254
Bouillon R et al (2022) The health effects of vitamin D supplementation: Evidence from human studies. Nat Rev Endocrinol 18:96–110
Wu H, Pang Q (2017) The effect of vitamin D and calcium supplementation on falls in older adults. Orthop 46:729–736
Liu C et al (2020) Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct 11:10817–10827
Grant AM et al (2005) Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): A randomised placebo-controlled trial. The Lancet 365:1621–1628
Smith LM, Gallagher JC, Kaufmann M, Jones G (2018) Effect of increasing doses of vitamin D on bone mineral density and serum N-terminal telopeptide in elderly women: A randomized controlled trial. J Intern Med 284:685–693
Ringe JD (2020) Plain vitamin D or active vitamin D in the treatment of osteoporosis: Where do we stand today? Arch Osteoporos 15:182
Uday S, Högler W (2017) Nutritional rickets and osteomalacia in the twenty-first century: Revised concepts, public health, and prevention strategies. Curr Osteoporos Rep 15:293–302
Bhambri R et al (2006) Changes in bone mineral density following treatment of osteomalacia. J Clin Densitom 9:120–127
Xu J, Yang Y, Ma L, Fu P, Peng H (2019) Cinacalcet plus vitamin D versus vitamin D alone for the treatment of secondary hyperparathyroidism in patients undergoing dialysis: A meta-analysis of randomized controlled trials. Int Urol Nephrol 51:2027–2036
Xing T, Hu Y, Wang B, Zhu J (2019) Role of oral calcium supplementation alone or with vitamin D in preventing post-thyroidectomy hypocalcaemia: A meta-analysis. Medicine (Baltimore) 98:e14455
Rendina D et al (2019) Vitamin D status in Paget disease of bone and efficacy–safety profile of cholecalciferol treatment in pagetic patients with hypovitaminosis D. Calcif Tissue Int 105:412–422
Merlotti D et al (2020) Preventive role of vitamin D supplementation for acute phase reaction after bisphosphonate infusion in Paget’s disease. J Clin Endocrinol Metab 105:e466–e476
van Lie Peters EM, Aronson DC, Everts V, Dooren LJ (1993) Failure of calcitriol treatment in a patient with malignant osteopetrosis. Eu. J Pediatr 152:818–821
Wu CC et al (2017) Diagnosis and management of osteopetrosis: Consensus guidelines from the osteopetrosis working group. J Clin Endocrinol Metab 102:3111–3123
Fusaro M et al (2020) Vitamin K and osteoporosis. Nutrients 12:3625
Araki S, Shirahata A (2020) Vitamin K deficiency bleeding in infancy. Nutrients 12:780
Cozzolino M, Fusaro M, Ciceri P, Gasperoni L, Cianciolo G (2019) The role of vitamin K in vascular calcification. Adv Chronic Kidney Dis 26:437–444
Evenepoel P et al (2019) Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res 34:262–269
Nakajima S et al (2011) Association of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel disease. Nutrition 27:1023–1028
Mott A et al (2019) Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int 30:1543–1559
Capozzi A, Scambia G, Lello S (2020) Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas 140:55–63
Omelka R et al (2021) The effects of eggshell calcium (Biomin H) and its combinations with alfacalcidol (1α-hydroxyvitamin D3) and menaquinone-7 (vitamin K2) on ovariectomy-induced bone loss in a rat model of osteoporosis. J Anim Physiol Anim Nutr 105:336–344
Bao M et al (2020) Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif 53:e12735
Kruger MC, Coetzee M, Haag M, Weiler H (2010) Long-chain polyunsaturated fatty acids: Selected mechanisms of action on bone. Prog Lipid Res 49:438–449
Sun D et al (2003) Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res 18:1206–1216
Watkins BA, Li Y, Seifert MF (2006) Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: Actions on bone mineral and serum biomarkers in ovariectomized rats. J Nutr Biochem 17:282–289
Zhang T et al (2021) Comparative study of DHA with different molecular forms for ameliorating osteoporosis by promoting chondrocyte-to-osteoblast transdifferentiation in the growth plate of ovariectomized mice. J Agric Food Chem 69:10562–10571
Atkinson TG, Barker HJ, Meckling-Gill KA (1997) Incorporation of long-chain n-3 fatty acids in tissues and enhanced bone marrow cellularity with docosahexaenoic acid feeding in post-weanling Fischer 344 rats. Lipids 32:293–302
Abdelhamid A et al (2019) The relationship between omega-3, omega-6 and total polyunsaturated fat and musculoskeletal health and functional status in adults: A systematic review and meta-analysis of RCTs. Calcif Tissue Int 105:353–372
Dou Y, Wang Y, Chen Z, Yu X, Ma D (2022) Effect of n-3 polyunsaturated fatty acid on bone health: A systematic review and meta-analysis of randomized controlled trials. Food Sci Nutr 10:145–154
Nemeth EF (2013) Allosteric modulators of the extracellular calcium receptor. Drug Discov Today Technol 10:e277–e284
Nemeth EF, Shoback D (2013) Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab 27:373–384
Nemeth EF (2008) Anabolic therapy for osteoporosis: Calcilytics. IBMS BoneKey 5:196–208
Langdahl BL (2021) Overview of treatment approaches to osteoporosis. Br J Pharmacol 178:1891–1906
Writing Group for the Women’s Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA 288:321–333
Shigehara K et al (2017) Effects of testosterone replacement therapy on hypogonadal men with osteopenia or osteoporosis: A subanalysis of a prospective randomized controlled study in Japan (EARTH study). Aging Male 20:139–145
Gagliano-Jucá T, Basaria S (2019) Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol 16:555–574
Chambers TJ, Magnus CJ (1982) Calcitonin alters behaviour of isolated osteoclasts. J Pathol 136:27–39
Chesnut CH et al (2008) Salmon calcitonin: A review of current and future therapeutic indications. Osteoporos Int 19:479–491
European Medicines Agency (EMA). Calcitonin approval status (2012). https://www.ema.europa.eu/en/medicines/human/referrals/calcitonin.
Lu J et al (2018) Advances in the discovery of cathepsin K inhibitors on bone resorption. J Enzyme Inhib Med Chem 33:890–904
Brixen K et al (2013) Bone density, turnover, and estimated strength in postmenopausal women treated with Odanacatib: A randomized trial. J Clin Endocrinol Metab 98:571–580
Eisman JA et al (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: Three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
McClung MR et al (2019) Odanacatib for the treatment of postmenopausal osteoporosis: Results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol 7:899–911
Kravets I (2018) Paget’s disease of bone: Diagnosis and treatment. Am J Med 131:1298–1303
Vestergaard P (2006) Current pharmacological options for the management of primary hyperparathyroidism. Drugs 66:2189–2211
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin Proc 83:1032–1045
European Medicine Agengy (EMA). EMA. Denosumab. https://www.ema.europa.eu/en/search/search?search_api_views_fulltext=denosumab (2023).
Dahiya N et al (2015) Denosumab: A bone antiresorptive drug. Med J Armed Forces India 71:71–75
Seeman E, Martin TJ (2019) Antiresorptive and anabolic agents in the prevention and reversal of bone fragility. Nat Rev Rheumatol 15:225–236
Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jørgensen NR (2012) Paget’s disease of the bone after treatment with Denosumab: A case report. Bone 50:1023–1025
Polyzos SA et al (2014) Denosumab treatment for juvenile Paget’s disease: Results from two adult patients with osteoprotegerin deficiency (“Balkan” mutation in the TNFRSF11B gene). J Clin Endocrinol Metab 99:703–707
Eller-Vainicher C et al (2018) Protective effect of Denosumab on bone in older women with primary hyperparathyroidism. J Am Geriatr Soc 66:518–524
Skjødt MK, Frost M, Abrahamsen B (2019) Side effects of drugs for osteoporosis and metastatic bone disease. Br J Clin Pharmacol 85:1063–1071
Diker-Cohen T et al (2020) Risk for infections during treatment with Denosumab for osteoporosis: A systematic review and meta-analysis. J Clin Endocrinol Metab 105:1641–1658
Zanchetta MB et al (2018) Significant bone loss after stopping long-term denosumab treatment: A post FREEDOM study. Osteoporos Int 29:41–47
Hwang JS et al (2008) The effects of strontium ranelate in Asian women with postmenopausal osteoporosis. Calcif Tissue Int 83:308–314
Khosla S, Hofbauer LC (2017) Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol 5:898–907
Reeve J et al (1976) Anabolic effect of low doses of a fragment of human parathyroid hormone on the skeleton in postmenopausal osteoporosis. The Lancet 307:1035–1038
Rouleau MF, Mitchell J, Goltzman D (1988) In vivo distribution of parathyroid hormone receptors in bone: Evidence that a predominant osseous target cell is not the mature osteoblast. Endocrinology 123:187–191
Isogai Y et al (1996) Parathyroid hormone regulates osteoblast differentiation positively or negatively depending on the differentiation stages. J Bone Miner Res 11:1384–1393
Tian Y, Xu Y, Fu Q, He M (2011) Parathyroid hormone regulates osteoblast differentiation in a Wnt/β-catenin-dependent manner. Mol Cell Biochem 355:211–216
Kousteni S, Bilezikian JP (2008) The cell biology of parathyroid hormone in osteoblasts. Curr Osteoporos Rep 6:72–76
Jilka RL (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446
Datta NS, Abou-Samra AB (2009) PTH and PTHrP signaling in osteoblasts. Cell Signal 21:1245–1254
Haas AV, LeBoff MS (2018) Osteoanabolic agents for osteoporosis. J Endocr Soc 2:922–932
Orwoll ES et al (2014) Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest 124:491–498
Tabacco G, Bilezikian JP (2019) Osteoanabolic and dual action drugs. Br J Clin Pharmacol 85:1084–1094
Jolette J et al (2017) Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH(1–34). Regul Toxicol Pharmacol 86:356–365
Gilsenan A et al (2021) Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res 36:244–251
Anastasilakis AD, Polyzos SA, Yavropoulou MP, Makras P (2020) Combination and sequential treatment in women with postmenopausal osteoporosis. Expert Opin Pharmacother 21:477–490
Lou S et al (2019) Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: A systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 30:59–70
Karner CM, Long F (2017) Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci 74:1649–1657
Miller SA, St Onge EL, Whalen KL (2021) Romosozumab: A novel agent in the treatment for postmenopausal osteoporosis. J Pharm Technol 37:45–52
Gennari L et al (2020) Emerging therapeutic targets for osteoporosis. Expert Opin Ther Targets 24:115–130
Sojan JM, Raman R, Muller M, Carnevali O, Renn J (2022) Probiotics enhance bone growth and rescue BMP inhibition: New transgenic zebrafish lines to study bone health. Int J Mol Sci 23:4748
Zemanova N, Omelka R, Mondockova V, Kovacova V, Martiniakova M (2022) Roles of gut microbiome in bone homeostasis and its relationship with bone-related diseases. Biology 11:1402
Maruyama M et al (2020) Modulation of the inflammatory response and bone healing. Front Endocrinol 11:386
Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT (2017) Oxidative stress in bone remodeling: Role of antioxidants. Clin Cases Miner Bone Metab 14:209–216
Tsukasaki M, Takayanagi H (2019) Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat Rev Immunol 19:626–642
Ko YJ et al (2021) A novel modified RANKL variant can prevent osteoporosis by acting as a vaccine and an inhibitor. Clin Transl Med 11:e368
Yang Y-S et al (2020) Bone-targeting AAV-mediated gene silencing in osteoclasts for osteoporosis therapy. Mol Ther-Methods Clin Dev 17:922–935
Han T-Y et al (2023) Bone targeted miRNA delivery system for miR-34a with enhanced anti-tumor efficacy to bone-associated metastatic breast cancer. Int J Pharm 635:122755
Jiang Y, Zhang P, Zhang X, Lv L, Zhou Y (2021) Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif 54:e12956
Lu C-H, Chen Y-A, Ke C-C, Liu R-S (2021) Mesenchymal stem cell-derived extracellular vesicle: A promising alternative therapy for osteoporosis. Int J Mol Sci 22:12750
Steward CG (2010) Hematopoietic stem cell transplantation for osteopetrosis. Pediatr Clin North Am 57:171–180
Stepensky P et al (2019) Stem cell transplantation for osteopetrosis in patients beyond the age of 5 years. Blood Adv 3:862–868
Even-Or E et al (2021) Haploidentical stem cell transplantation with post-transplant cyclophosphamide for osteopetrosis and other nonmalignant diseases. Bone Marrow Transplant 56:434–441
Löfvall H (2019) Hematopoietic stem cell-targeted neonatal gene therapy with a clinically applicable lentiviral vector corrects osteopetrosis in oc/oc mice. Hum Gene Ther 30:1395–1404
Shen B (2015) A New Golden age of natural products drug discovery. Cell 163:1297–1300
Li F, Wang Y, Li D, Chen Y, Dou QP (2019) Are we seeing a resurgence in the use of natural products for new drug discovery? Expert Opin Drug Discov 14:417–420
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803
Hu Y et al (2015) Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar Drugs 13:202–221
Calixto JB (2019) The role of natural products in modern drug discovery. An Acad Bras Cienc 91:e20190105
Martiniakova M, Babikova M, Omelka R (2020) Pharmacological agents and natural compounds: Available treatments for osteoporosis. J Physiol Pharmacol 71:307–320
Zhao H et al (2018) Prevention and treatment of osteoporosis using chinese medicinal plants: Special emphasis on mechanisms of immune modulation. J Immunol Res 2018:6345857
Blunt W, Copp JR, Keyzers BA, Munro RG, Prinsep MHMR (2017) Marine natural products. Nat Prod Rep 34:235–294
Senthilkumar K, Venkatesan J, Kim S-K (2014) Marine derived natural products for osteoporosis. Biomed Prev Nutr 4:1–7
Clarke SA, Walsh P (2014) Marine organisms for bone repair and regeneration. In: Mallick K (ed) Bone Substitute Biomaterials. Woodhead Publishing, UK, pp 294–318
Clarke SA, Walsh P, Maggs CA, Buchanan F (2011) Designs from the deep: Marine organisms for bone tissue engineering. Biotechnol Adv 29:610–617
John M, Sugunan A, Aswathy S, Revu DS (2023) Marine based biomaterials: A Marvel in periodontal regeneration – A Review. Adv Dent J 5:24–34
Wang Z et al (2023) Current application and modification strategy of marine polysaccharides in tissue regeneration: A review. Biomater Adv 154:213580
Ahn KS et al (2007) Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-κB–regulated gene products. Blood 110:2286–2295
Yonezawa T et al (2012) Biselyngbyaside, isolated from marine cyanobacteria, inhibits osteoclastogenesis and induces apoptosis in mature osteoclasts. J Cell Biochem 113:440–448
Yamano A et al (2020) Irijimasides A-E, macrolide glycosides from an Okeania sp. marine cyanobacterium. J Nat Prod 83:1585–1591
Sapkota M, Li L, Choi H, Gerwick WH, Soh Y (2015) Bromo-honaucin A inhibits osteoclastogenic differentiation in RAW 264.7 cells via Akt and ERK signaling pathways. Eur J Pharmacol 769:100–109
Li L et al (2021) Kalkitoxin reduces osteoclast formation and resorption and protects against inflammatory bone loss. Int J Mol Sci 22:2303
Kita M et al (2004) Symbioimine exhibiting inhibitory effect of osteoclast differentiation, from the symbiotic marine dinoflagellate Symbiodinium sp. J Am Chem Soc 126:4794–4795
Kim SC et al (2022) Sulfated glucuronorhamnoxylan from Capsosiphon fulvescens ameliorates osteoporotic bone resorption via inhibition of osteoclastic cell differentiation and function in vitro and in vivo. Mar Biotechnol 24:690–705
Das SK, Ren R, Hashimoto T, Kanazawa K (2010) Fucoxanthin induces apoptosis in osteoclast-like cells differentiated from RAW264.7 cells. J Agric Food Chem 58:6090–6095
Ha Y-J et al (2021) Fucoxanthin suppresses osteoclastogenesis via modulation of MAP kinase and Nrf2 signaling. Mar Drugs 19:132
Guo L et al (2020) Protective effect of fucoxanthin on ovariectomy-induced osteoporosis in rats. Pharmacogn Mag 16:242–249
Yoon W-J et al (2013) Sargachromanol G inhibits osteoclastogenesis by suppressing the activation NF-κB and MAPKs in RANKL-induced RAW 264.7 cells. Biochem Biophys Res Commun 434:892–897
Jin W, Chen F, Fang Q, Mao G, Bao Y (2023) Oligosaccharides from Sargassum thunbergii inhibit osteoclast differentiation via regulation of IRF-8 signaling. Exp Gerontol 172:112057
Zhu J et al (2013) Mycoepoxydiene suppresses RANKL-induced osteoclast differentiation and reduces ovariectomy-induced bone loss in mice. Appl Microbiol Biotechnol 97:767–774
Kim JW et al (2016) Stachybotrysin, an osteoclast differentiation inhibitor from the marine-derived fungus Stachybotrys sp. KCB13F013. J Nat. Prod 79:2703–2708
Wang J et al (2018) Osteoclastogenesis inhibitory polyketides from the sponge-associated fungus Xylaria feejeensis. Chem Biodivers 15:e1800358
Liu D-H et al (2019) Osteoclastogenesis regulation metabolites from the coral-associated fungus Pseudallescheria boydii TW-1024-3. J Nat Prod 82:1274–1282
Hu Y et al (2023) New meroterpenoids and anti-osteoclastogenic polyketides from the mangrove-derived fungus Arthrinium sp. SCSIO 41306. Chem Biodivers 20:e202300551
Tan Y et al (2020) A marine fungus-derived nitrobenzoyl sesquiterpenoid suppresses receptor activator of NF-κB ligand-induced osteoclastogenesis and inflammatory bone destruction. Br J Pharmacol 177:4242–4260
Kato H et al (2017) Enantioselective inhibitory abilities of enantiomers of notoamides against RANKL-induced formation of multinuclear osteoclasts. Bioorg Med Chem Lett 27:4975–4978
Wang W et al (2019) Austalides, Osteoclast differentiation inhibitors from a marine-derived strain of the Fungus Penicillium rudallense. J Nat Prod 82:3083–3088
Zhang Y et al (2022) Anti-osteoclastogenic and antibacterial effects of chlorinated polyketides from the Beibu gulf coral-derived fungus Aspergillus unguis GXIMD 02505. Mar Drugs 20:178
El-Desoky AHH et al (2021) Taichunins E-T, isopimarane diterpenes and a 20-nor-isopimarane, from Aspergillus taichungensis (IBT 19404): Structures and inhibitory effects on RANKL-induced formation of multinuclear osteoclasts. J Nat Prod 84:2475–2485
Shin HJ et al (2018) Suppression of RANKL-induced osteoclastogenesis by the metabolites from the marine fungus Aspergillus flocculosus isolated from a sponge Stylissa sp. Mar Drugs 16:14
Song Y et al (2023) Tanzawaic acid derivatives from the marine-derived Penicillium steckii as inhibitors of RANKL-induced osteoclastogenesis. J Nat Prod 86:1171–1178
Kang MR et al (2014) Agelasine D suppresses RANKL-induced osteoclastogenesis via down-regulation of c-Fos, NFATc1 and NF-κB. Mar Drugs 12:5643–5656
Kim H et al (2014) Placotylene A, an inhibitor of the receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation, from a Korean sponge Placospongia sp. Mar Drugs 12:2054–2065
Tsukamoto S et al (2014) Halenaquinone inhibits RANKL-induced osteoclastogenesis. Bioorg Med Chem Lett 24:5315–5317
Patil AD et al (2002) Haploscleridamine, a novel tryptamine-derived alkaloid from a sponge of the order Haplosclerida: An inhibitor of cathepsin K. J Nat Prod 65:628–629
El-Desoky AH et al (2016) Ceylonamides A-F, nitrogenous spongian diterpenes that inhibit RANKL-induced osteoclastogenesis, from the marine sponge Spongia ceylonensis. J Nat Prod 79:1922–1928
El-Desoky AH et al (2017) Ceylonins A-F, spongian diterpene derivatives that inhibit RANKL-induced formation of multinuclear osteoclasts, from the marine sponge Spongia ceylonensis. J Nat Prod 80:90–95
Tsukamoto S et al (2018) Isolation of aaptic acid from the marine sponge Aaptos lobata and inhibitory effect of aaptamines on RANKL-induced formation of multinuclear osteoclasts. Heterocycles 97:1219–1225
El-Beih AA et al (2018) New inhibitors of RANKL-induced osteoclastogenesis from the marine sponge Siphonochalina siphonella. Fitoterapia 128:43–49
Maeyama Y et al (2021) Amakusamine from a Psammocinia sp. sponge: Isolation, synthesis, and SAR study on the inhibition of RANKL-induced formation of multinuclear osteoclasts. J Nat Prod 84:2738–2743
El-Desoky AH et al (2023) Aaptocarbamates A−G, chlorinated terpene carbamates with antiosteoclastogenic activities from the marine sponge Aaptos sp. Phytochemistry 216:113872
Lin Y-Y et al (2013) A soft coral-derived compound, 11-epi-sinulariolide acetate suppresses inflammatory response and bone destruction in adjuvant-induced arthritis. PLoS One 8:e62926
Meng J et al (2021) Briarane-type diterpenoids suppress osteoclastogenisis by regulation of Nrf2 and MAPK/NF-kB signaling pathway. Bioorganic Chem 112:104976
Lin Y-Y et al (2017) Excavatolide B attenuates rheumatoid arthritis through the inhibition of isteoclastogenesis. Mar Drugs 15:9
Lu H et al (2022) Osteoclastogenesis inhibitory phenolic derivatives produced by the Beibu Gulf coral-associated fungus Acremonium sclerotigenum GXIMD 02501. Fitoterapia 159:105201
Xu C, Li J, Su L, Tang H, Zhang W (2020) Osteoclastogenesis modulatory steroids from the South China Sea gorgonian coral Iciligorgia sp. Chem Biodivers 17:e2000266
Qi X et al (2023) Briarane-type diterpenoids, the inhibitors of osteoclast formation by interrupting Keap1-Nrf2 interaction and activating Nrf2 pathway. Eur J Med Chem 246:114948
Kazami S et al (2006) Iejimalides show anti-osteoclast activity via V-ATPase inhibition. Biosci Biotechnol Biochem 70:1364–1370
He Z-H et al (2023) Neotricitrinols A-C, unprecedented citrinin trimers with anti-osteoporosis activity from the deep-sea-derived Penicillium citrinum W23. Bioorganic Chem 139:106756
Xie C-L et al (2023) Deep-sea-derived Penicopeptide a (Ppa) promotes osteoblast-mediated bone formation and alleviates ovariectomy-induced bone loss by activating the Akt/Gsk-3β/Β-Catenin signaling pathway. SSRN Scholarly Paper at. https://doi.org/10.2139/ssrn.4429292
Kim SN et al (2012) In vitro and in vivo osteogenic activity of licochalcone A. Amino Acids 42:1455–1465
Natsume N, Ozaki K, Nakajima D, Yokoshima S, Teruya T (2020) Structure–activity relationship study of Majusculamides A and B and their analogues on osteogenic activity. J Nat Prod 83:2477–2482
Akakabe M et al (2014) Amphirionin-5, a novel linear polyketide from a cultured marine dinoflagellate Amphidinium species with a potent cell proliferation-promoting activity. Tetrahedron Lett 55:3491–3494
Ryu B, Li Y-X, Kang K-H, Kim S-K, Kim DG (2015) Floridoside from Laurencia undulata promotes osteogenic differentiation in murine bone marrow mesenchymal cells. J Funct Foods 19:505–511
Chen Y et al (2021) Dunaliella salina-derived peptide protects from bone loss: Isolation, purification and identification. LWT 137:110437
Nguyen MHT et al (2013) Tetrameric peptide purified from hydrolysates of biodiesel byproducts of Nannochloropsis oculata induces osteoblastic differentiation through MAPK and Smad pathway on MG-63 and D1 cells. Process Biochem 48:1387–1394
Ryu B, Li Y, Qian Z-J, Kim M-M, Kim S-K (2009) Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem Biol Interact 179:192–201
Ahn B-N et al (2016) Dioxinodehydroeckol enhances the differentiation of osteoblasts by regulating the expression of phospho-Smad1/5/8. Mar Drugs 14:168
Kim J-A et al (2016) Bioactive quinone derivatives from the marine brown alga Sargassum thunbergii induce anti-adipogenic and pro-osteoblastogenic activities. J Sci Food Agric 96:783–790
Oh JH et al (2019) Phlorofucofuroeckol A from edible brown alga Ecklonia Cava enhances osteoblastogenesis in bone marrow-derived human mesenchymal stem cells. Mar Drugs 17:543
Byun MR et al (2012) Phorbaketal A stimulates osteoblast differentiation through TAZ mediated Runx2 activation. FEBS Lett 586:1086–1092
Rho JR et al (2011) Phorbasones A and B, sesterterpenoids isolated from the marine sponge Phorbas sp. and induction of osteoblast differentiation. Org. Lett. 13:884–887
Carnovali M et al (2022) Aerophobin-1 from the marine sponge Aplysina aerophoba modulates osteogenesis in zebrafish larvae. Mar Drugs 20:135
Kinugawa M, Fukuzawa S, Tachibana K (2009) Skeletal protein protection: The mode of action of an anti-osteoporotic marine alkaloid, norzoanthamine. J Bone Miner Metab 27:303–314
Cuong NX et al (2008) New cembranoid diterpenes from the Vietnamese soft coral Sarcophyton mililatensis stimulate osteoblastic differentiation in MC3T3-E1 cells. Chem Pharm Bull (Tokyo) 56:988–992
Van Minh C et al (2007) A new 9,11-secosterol from the Vietnamese sea soft coral, Sarcophyton mililatensis, increases the function of osteoblastic MC3T3-E1 cells. Nat Prod Commun 2:1934578X070020109
Oh Y, Ahn C-B, Je J-Y (2020) Blue mussel-derived peptides PIISVYWK and FSVVPSPK trigger Wnt/β-Catenin signaling-mediated osteogenesis in human bone marrow mesenchymal stem cells. Mar Drugs 18:510
Oh Y, Ahn C-B, Cho WH, Yoon NY, Je J-Y (2020) Anti-osteoporotic effects of antioxidant peptides PIISVYWK and FSVVPSPK from Mytilus edulis on ovariectomized mice. Antioxidants 9:866
Chen H et al (2019) Identification and mechanism evaluation of a novel osteogenesis promoting peptide from Tubulin Alpha-1C chain in Crassostrea gigas. Food Chem 272:751–757
Guo J, Liao J, Li YP, Song WD, Liu JS (2013) Study on anti-osteoporosis of compound pearl protein polypeptide. Adv Mater Res 781–784:1260–1264
Jiang Z et al (2018) Dietary natural N-acetyl-D-glucosamine prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. Molecules 23:2302
Wang P et al (2023) A synthetic peptide from Sipunculus nudus promotes bone formation via Estrogen/MAPK signal pathway based on network pharmacology. Front Pharmacol 14:1173110
Wang Y et al (2021) Stichopus japonicus polysaccharide stimulates osteoblast differentiation through activation of the bone morphogenetic protein pathway in MC3T3-E1 cells. J Agric Food Chem 69:2576–2584
Lee Y-S, Feng C-W, Peng M-Y, Chen Y-C, Chan T-F (2022) Antiosteoporosis effects of a marine antimicrobial peptide pardaxin via regulation of the osteogenesis pathway. Peptides 148:170686
Heo S-Y et al (2018) Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem Funct 36:137–146
Gavva C, Patel K, Kudre T, Sharan K, Chilkunda DN (2020) Glycosaminoglycans from fresh water fish processing discard - Isolation, structural characterization, and osteogenic activity. Int J Biol Macromol 145:558–567
Nguyen V-T et al (2018) Ciona intestinalis calcitonin-like peptide promotes osteoblast differentiation and mineralization through MAPK pathway in MC3T3-E1 cells. Process Biochem 67:127–138
Li L, Sapkota M, Gao M, Choi H, Soh Y (2017) Macrolactin F inhibits RANKL-mediated osteoclastogenesis by suppressing Akt, MAPK and NFATc1 pathways and promotes osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway. Eur J Pharmacol 815:202–209
Sapkota M et al (2020) Macrolactin A protects against LPS-induced bone loss by regulation of bone remodeling. Eur J Pharmacol 883:173305
Jin X et al (2017) Low-molecular weight fucoidan inhibits the differentiation of osteoclasts and reduces osteoporosis in ovariectomized rats. Mol Med Rep 15:890–898
Cho Y-S, Jung W-K, Kim J-A, Choi I-W, Kim S-K (2009) Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem 116:990–994
Lee D-G et al (2014) The bone regenerative effects of fucosterol in in vitro and in vivo models of postmenopausal osteoporosis. Mol Nutr Food Res 58:1249–1257
Ihn HJ et al (2017) Diphlorethohydroxycarmalol from Ishige okamurae suppresses osteoclast differentiation by downregulating the NF-κB signaling pathway. Int J Mol Sci 18:2635
Lee S-H, Kim M, Park MH (2021) Diphlorethohydroxycamalol isolated from Ishige okamurae prevents H2O2-induced oxidative damage via BMP2/Runx2 signaling in osteoblastic MC3T3-E1 cells. Fitoterapia 152:104921
Li W, Haiya W, Ningyuan F (2016) Algal oligosaccharides ameliorate osteoporosis via up-regulation of parathyroid hormone 1–84 and vascular endothelial growth factor. J Tradit Chin Med 36:332–339
Cho S-H et al (2023) Effect of Ishophloroglucin A isolated from Ishige okamurae on in vitro osteoclastogenesis and osteoblastogenesis. Mar Drugs 21:377
Hwang Y-H et al (2018) Suppression effect of astaxanthin on osteoclast formation in vitro and bone loss in vivo. Int J Mol Sci 19:912
Zhao G, Zhong H, Rao T, Pan Z (2020) Metabolomic analysis reveals that the mechanism of astaxanthin improves the osteogenic differentiation potential in bone marrow mesenchymal stem cells. Oxid Med Cell Longev 2020:e3427430
Kim K-J et al (2021) Austalide K from the fungus Penicillium rudallense prevents LPS-induced bone loss in mice by inhibiting osteoclast differentiation and promoting osteoblast differentiation. Int J Mol Sci 22:5493
Wang Q et al (2020) Hymenialdisine: A marine natural product that acts on both osteoblasts and osteoclasts and prevents estrogen-dependent bone loss in mice. J Bone Miner Res 35:1582–1596
Xu Z et al (2019) Bone formation activity of an osteogenic dodecapeptide from blue mussels (Mytilus edulis). Food Funct 10:5616–5625
Xu Z et al (2020) Pharmacokinetics and transport of an osteogenic dodecapeptide. J Agric Food Chem 68:9961–9967
Song J et al (2022) Positive effect of compound amino acid chelated calcium from the shell and skirt of scallop in an ovariectomized rat model of postmenopausal osteoporosis. J Sci Food Agric 102:1363–1371
Lee D-W et al (2019) The effect of polydeoxyribonucleotide extracted from salmon sperm on the restoration of bisphosphonate-related osteonecrosis of the jaw. Mar Drugs 17:51
Menaa F et al (2021) Marine algae-derived bioactive compounds: A new wave of nanodrugs? Mar Drugs 19:484
Brennan O et al (2017) A natural calcium-rich multi-mineral complex preserves bones structure, composition and strength in an ovariectomized rat model of osteoporosis. Calcif Tissue Int 101:445–455
Carson MA et al (2018) Red algal extracts from Plocamium lyngbyanum and Ceramium secundatum stimulate osteogenic activities in vitro and bone growth in zebrafish larvae. Sci Rep 8:7725
Nekooei M, Shafiee SM, Zahiri M, Maryamabadi A, Nabipour I (2021) The methanol extract of red algae, Dichotomaria obtusata, from Persian Gulf promotes in vitro osteogenic differentiation of bone marrow mesenchymal stem cells: A biological and phytochemical study. J Pharm Pharmacol 73:347–356
Minetti M et al (2019) Padina pavonica extract promotes in vitro differentiation and functionality of human primary osteoblasts. Mar Drugs 17:473
Yu H, Chen Y, Zhu J (2022) Osteogenic activities of four calcium-chelating microalgae peptides. J Sci Food Agric 102:6643–6649
Balasubramaniam V, Gunasegavan RD-N, Mustar S, Lee JC, Mohd Noh MF (2021) Isolation of industrial important bioactive compounds from microalgae. Molecules 26:943
Shi Q et al (2021) Transgenic eukaryotic microalgae as green factories: Providing new ideas for the production of biologically active substances. J Appl Phycol 33:705–728
Carletti A et al (2023) The osteogenic and mineralogenic potential of the microalgae Skeletonema costatum and Tetraselmis striata CTP4 in fish models. Cell Mol Life Sci 80:310
Grienke U, Silke J, Tasdemir D (2014) Bioactive compounds from marine mussels and their effects on human health. Food Chem 142:48–60
Green DW, Kwon H-J, Jung H-S (2015) Osteogenic potency of nacre on human mesenchymal stem cells. Mol Cells 38:267–272
Duplat D et al (2007) The effect of molecules in mother-of-pearl on the decrease in bone resorption through the inhibition of osteoclast cathepsin K. Biomaterials 28:4769–4778
Molagoda IMN et al (2019) Fermented oyster extract promotes osteoblast differentiation by activating the Wnt/β-Catenin signaling pathway, leading to bone formation. Biomolecules 9:711
Molagoda IMN et al (2020) Fermented oyster extract promotes insulin-like growth factor-1-mediated osteogenesis and growth Rate. Mar Drugs 18:472
Ihn HJ et al (2019) Fermented oyster extract prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis. Nutrients 11:1392
Choi MH, Lee K, Kim MY, Shin H-I, Jeong D (2019) Pisidium coreanum inhibits multinucleated osteoclast formation and prevents estrogen-deficient osteoporosis. Int J Mol Sci 20:6076
Chaugule S et al (2019) Hexane fraction of Turbo brunneus inhibits intermediates of RANK-RANKL signaling pathway and prevent ovariectomy induced bone loss. Front Endocrinol 10:608
Balakrishnan B, Chiplunkar SV, Indap MM (2014) Methanol extract of Euchelus asper prevents bone resorption in ovariectomised mice model. J Osteoporos 2014:e348189
Hou Y et al (2020) PHNQ from Evechinus chloroticus sea urchin supplemented with calcium promotes mineralization in SaOS-2 human bone cell line. Mar Drugs 18:373
Shahrulazua A, Samsudin A, Iskandar M, Amran A (2013) The in-vitro effects of sea cucumber (Stichopus sp1) extract on human osteoblast cell line. Malays Orthop J 7:41–48
Chen Z et al (2022) Sea cucumber enzymatic hydrolysates relieve osteoporosis through OPG/RANK/RANKL system in ovariectomized rats. Food Biosci 46:101572
Arumugam V, Venkatesan M, Ramachandran S, Sundaresan U (2018) Bioactive peptides from marine ascidians and future drug development: A review. Int J Pept Res Ther 24:13–18
Carletti A et al (2022) Antioxidant and anti-inflammatory extracts from sea cucumbers and tunicates induce a pro-osteogenic effect in zebrafish larvae. Front Nutr 9:888360
Huang R, Rong Q, Han X, Li Y (2015) The effects of cod bone gelatin on trabecular microstructure and mechanical properties of cancellous bone. Acta Mech Solida Sin 28:1–10
Nomura Y, Oohashi K, Watanabe M, Kasugai S (2005) Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition 21:1120–1126
Li J et al (2020) Tuna bone powder alleviates glucocorticoid-induced osteoporosis via coregulation of the NF-κB and Wnt/β-Catenin signaling pathways and modulation of gut microbiota composition and metabolism. Mol Nutr Food Res 64:1900861
Karadeniz F, Kim J-A, Ahn B-N, Kwon MS, Kong C-S (2014) Effect of Salicornia herbacea on osteoblastogenesis and adipogenesis in vitro. Mar Drugs 12:5132–5147
Karadeniz F, Kim J-A, Ahn B-N, Kim M, Kong C-S (2014) Anti-adipogenic and pro-osteoblastogenic activities of Spergularia marina extract. Prev Nutr Food Sci 19:187–193
Roberto VP et al (2021) Antioxidant, mineralogenic and osteogenic activities of Spartina alterniflora and Salicornia fragilis extracts rich in polyphenols. Front Nutr 8:719438
Canales CP, Walz K (2019) The mouse, a model organism for biomedical research. In: Walz K, Young JI (eds) Cellular and Animal Models in Human Genomics Research. Academic Press, UK, pp 119–140
Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A (2020) Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J 19:89–107
Halade GV, Rahman MM, Williams PJ, Fernandes G (2010) High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem 21:1162–1169
Sophocleous A, Idris AI (2014) Rodent models of osteoporosis. BoneKEy Rep 3:614
Xavier A, Toumi H, Lespessailles E (2022) Animal model for glucocorticoid induced osteoporosis: A systematic review from 2011 to 2021. Int J Mol Sci 23:377
Kim J-W, Tatad JCI, Landayan MEA, Kim S-J, Kim M-R (2015) Animal model for medication-related osteonecrosis of the jaw with precedent metabolic bone disease. Bone 81:442–448
Eisman JA, Bouillon R (2014) Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. BoneKEy Rep 3:499
Lotinun S, Sibonga JD, Turner RT (2003) Triazolopyrimidine (Trapidil), a platelet-derived growth factor antagonist, inhibits parathyroid bone disease in an animal model for chronic hyperparathyroidism. Endocrinology 144:2000–2007
Ni L-H et al (2018) A rat model of SHPT with bone abnormalities in CKD induced by adenine and a high phosphorus diet. Biochem Biophys Res Commun 498:654–659
Wei Z et al (2021) Mutations in Profilin 1 cause early-onset Paget’s disease of bone with giant cell tumors. J Bone Miner Res 36:1088–1103
Alonso N et al (2021) Insertion mutation in Tnfrsf11a causes a Paget’s disease–like phenotype in heterozygous mice and osteopetrosis in homozygous mice. J Bone Miner Res 36:1376–1386
Lleras-Forero L, Winkler C, Schulte-Merker S (2020) Zebrafish and medaka as models for biomedical research of bone diseases. Dev Biol 457:191–205
Dietrich K et al (2021) Skeletal biology and disease modeling in zebrafish. J Bone Miner Res 36:436–458
Rosa JT, Tarasco M, Gavaia PJ, Cancela ML, Laizé V (2022) Screening of mineralogenic and osteogenic compounds in zebrafish—Tools to improve assay throughput and data accuracy. Pharmaceuticals 15:983
Brittijn SA et al (2009) Zebrafish development and regeneration: New tools for biomedical research. Int J Dev Biol 53:835–850
Laizé V, Gavaia PJ, Cancela ML (2014) Fish: A suitable system to model human bone disorders and discover drugs with osteogenic or osteotoxic activities. Drug Discov Today Dis Models 13:29–37
Rosa JT, Laizé V, Gavaia PJ, Cancela ML (2021) Fish models of induced osteoporosis. Front Cell Dev Biol 9:672424
To TT, Witten PE, Huysseune A, Winkler C (2015) An adult osteopetrosis model in medaka reveals the importance of osteoclast function for bone remodeling in teleost fish. Comp Biochem Physiol Part C Toxicol Pharmacol 178:68–75
Silva IAL, Conceição N, Michou L, Cancela ML (2014) Can zebrafish be a valid model to study Paget’s disease of bone? J Appl Ichthyol 30:678–688
Dubale NM, Kapron CM, West SL (2022) Commentary: Zebrafish as a model for osteoporosis—An approach to accelerating progress in drug and exercise-based treatment. Int J Environ Res Public Health 19:15866
Surget G et al (2017) Marine green macroalgae: A source of natural compounds with mineralogenic and antioxidant activities. J Applied Phycol 29:575–584
Acknowledgements
This work was financed by the European Maritime and Fisheries Fund (EMFF/FEAMP) through the National Operational Programme MAR2020 (grant 16-02-01-FMP-0057/OSTEOMAR), by the European Regional Development Fund (ERDF/FEDER) through the Transnational Cooperation Programme Atlantic Area (grant EAPA/151/2016/BLUEHUMAN), by the Marie Skłodowska-Curie innovative training network BIOMEDAQU (grant H2020-MSCA-ITN/766347), by National funds through the Portuguese Foundation for Science and Technology (grants UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020, and doctoral fellowships 2021.05406.BD and SFRH/BD/140143/2018) and by the operational programs CRESC Algarve 2020 and COMPETE 2020 through project EMBRC.PT ALG-01-0145-FEDER-022121.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
All authors were responsible for the conceptualization of the manuscript. AC was responsible for investigation, methodology, data curation, and writing of the first draft of the manuscript. AC, VL, PJG, and MLC, were responsible for editing and review. PJG, MLC, and VL were responsible for resources, supervision, and funding acquisition. All authors approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethical approval
No animals were used in the present study.
Consent for publication
No human research participants were involved in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carletti, A., Gavaia, P.J., Cancela, M.L. et al. Metabolic bone disorders and the promise of marine osteoactive compounds. Cell. Mol. Life Sci. 81, 11 (2024). https://doi.org/10.1007/s00018-023-05033-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-05033-x