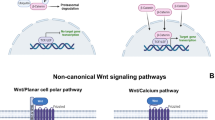
Abstract
The adult human skeleton is a multifunctional organ undergoing continuous remodeling. Homeostasis of bone mass in a healthy adult requires an exquisite balance between bone resorption by osteoclasts and bone formation by osteoblasts; disturbance of such balance is the root cause for various bone disorders including osteoporosis. To develop effective and safe therapeutics to modulate bone formation, it is essential to elucidate the molecular mechanisms governing osteoblast differentiation and activity. Due to their specialized function in collagen synthesis and secretion, osteoblasts are expected to consume large amounts of nutrients. However, studies of bioenergetics and building blocks in osteoblasts have been lagging behind those of growth factors and transcription factors. Genetic studies in both humans and mice over the past 15 years have established Wnt signaling as a critical mechanism for stimulating osteoblast differentiation and activity. Importantly, recent studies have uncovered that Wnt signaling directly reprograms cellular metabolism by stimulating aerobic glycolysis, glutamine catabolism as well as fatty acid oxidation in osteoblast-lineage cells. Such findings therefore reveal an important regulatory axis between bone anabolic signals and cellular bioenergetics. A comprehensive understanding of osteoblast metabolism and its regulation is likely to reveal molecular targets for novel bone therapies.


Similar content being viewed by others
References
Long F (2012) Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 13(1):27–38
Karsenty G, Kronenberg HM, Settembre C (2009) Genetic control of bone formation. Annu Rev Cell Dev Biol 25:629–648
Ducy P et al (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89(5):747–754
Lee B et al (1997) Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet 16(3):307–310
Komori T et al (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89(5):755–764
Nakashima K et al (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108(1):17–29
Yang X et al (2004) ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell 117(3):387–398
Zhong Z, Ethen NJ, Williams BO (2014) WNT signaling in bone development and homeostasis. Wiley Interdiscip Rev Dev Biol 3(6):489–500
Buttgereit F, Brand MD (1995) A hierarchy of ATP-consuming processes in mammalian cells. Biochem J 312(Pt 1):163–167
Bennett CN et al (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102(9):3324–3329
Tu X et al (2007) Noncanonical Wnt signaling through G protein-Linked PKCdelta activation promotes bone formation. Dev Cell 12(1):113–127
Day TF et al (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8(5):739–750
Hu H et al (2005) Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132(1):49–60
Babij P et al (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18(6):960–974
Gong Y et al (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107(4):513–523
Rodda SJ, McMahon AP (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133(16):3231–3244
Lu W et al (2004) Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119(1):97–108
He X et al (2004) LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131(8):1663–1677
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Kimelman D, Xu W (2006) Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25(57):7482–7491
Taelman VF et al (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143(7):1136–1148
Behrens J et al (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382(6592):638–642
Hovanes K et al (2001) Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 28(1):53–57
Zeng W et al (2000) naked cuticle encodes an inducible antagonist of Wnt signalling. Nature 403(6771):789–795
Roose J et al (1999) Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285(5435):1923–1926
Jho EH et al (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22(4):1172–1183
Chen AE, Ginty DD, Fan CM (2005) Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature 433(7023):317–322
Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 17(2):295–309
Inoki K et al (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126(5):955–968
Sheldahl LC et al (1999) Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 9(13):695–698
Sheldahl LC et al (2003) Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 161(4):769–777
Wu X et al (2008) Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133(2):340–353
Yamanaka H et al (2002) JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3(1):69–75
Hino S et al (2001) Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein. Mol Cell Biol 21(1):330–342
Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4(4):e115
Simons M et al (2005) Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37(5):537–543
van Amerongen R et al (2012) Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol 369(1):101–114
Mao B et al (2001) LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411(6835):321–325
Semenov MV et al (2001) Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11(12):951–961
Semenov M, Tamai K, He X (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280(29):26770–26775
Bourhis E et al (2011) Wnt antagonists bind through a short peptide to the first beta-propeller domain of LRP5/6. Structure 19(10):1433–1442
Rattner A et al (1997) A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA 94(7):2859–2863
Finch PW et al (1997) Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA 94(13):6770–6775
Leyns L et al (1997) Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88(6):747–756
Maupin KA, Droscha CJ, Williams BO (2013) A comprehensive overview of skeletal phenotypes associated with alterations in Wnt/b-catenin signaling in humans and mice. Bone Res 1(1):27–71
Boyden LM et al (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346(20):1513–1521
Little RD et al (2002) A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70(1):11–19
van Bezooijen RL et al (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199(6):805–814
Brunkow ME et al (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68(3):577–589
Balemans W et al (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39(2):91–97
Richards JB et al (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371(9623):1505–1512
Rivadeneira F et al (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41(11):1199–1206
van Meurs JB et al (2008) Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299(11):1277–1290
Estrada K et al (2012) Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 44(5):491–501
Fahiminiya S et al (2013) Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet 50(5):345–348
Laine CM et al (2013) WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med 368(19):1809–1816
Zheng HF et al (2012) WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet 8(7):e1002745
Kato M et al (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157(2):303–314
Riddle RC et al (2013) Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS One 8(5):e63323
Cui Y et al (2011) Lrp5 functions in bone to regulate bone mass. Nat Med 17(6):684–691
Li X et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23(6):860–869
Morvan F et al (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21(6):934–945
Bodine PV et al (2004) The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol 18(5):1222–1237
Albers J et al (2013) Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol 200(4):537–549
Albers J et al (2011) Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol 192(6):1057–1072
Joeng KS et al (2014) The swaying mouse as a model of osteogenesis imperfecta caused by WNT1 mutations. Hum Mol Genet 23(15):4035–4042
Maruyama T, Jiang M, Hsu W (2013) Gpr177, a novel locus for bone mineral density and osteoporosis, regulates osteogenesis and chondrogenesis in skeletal development. J Bone Miner Res 28(5):1150–1159
Zhong Z et al (2012) Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci USA 109(33):E2197–E2204
Witte F et al (2009) Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns 9(4):215–223
Chen J, Long F (2013) beta-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res 28(5):1160–1169
Glass DA 2nd et al (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8(5):751–764
Hill TP et al (2005) Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8(5):727–738
Holmen SL et al (2005) Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 280(22):21162–21168
Song L et al (2012) Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res 27(11):2344–2358
Gaur T et al (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280(39):33132–33140
Chen J et al (2014) WNT7B promotes bone formation in part through mTORC1. PLoS Genet 10(1):e1004145
Karner CM et al (2015) Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Investig 125(2):551–562
Riddle RC et al (2014) Tsc2 is a molecular checkpoint controlling osteoblast development and glucose homeostasis. Mol Cell Biol 34(10):1850–1862
Chen J, Long F (2015) mTORC1 signaling promotes osteoblast differentiation from preosteoblasts. PLoS One 10(6):e0130627
Sun W et al (2016) Rictor is required for optimal bone accrual in response to anti-sclerostin therapy in the mouse. Bone 85:1–8
Esen E et al (2013) WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab 17(5):745–755
Chen J et al (2015) mTORC2 signaling promotes skeletal growth and bone formation in mice. J Bone Miner Res 30:369–378
Bell GI et al (1993) Structure and function of mammalian facilitative sugar transporters. J Biol Chem 268(26):19161–19164
Mueckler M (1994) Facilitative glucose transporters. Eur J Biochem 219(3):713–725
Esen E, Long F (2014) Aerobic glycolysis in osteoblasts. Curr Osteoporos Rep 12(4):433–438
Tsukada Y et al (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 439(7078):811–816
Tahiliani M et al (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324(5929):930–935
Wellen KE et al (2009) ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324(5930):1076–1080
Brommage R, Neuman WF (1979) Mechanism of mobilization of bone mineral by 1,25-dihydroxyvitamin D3. Am J Physiol 237(2):E113–E120
Cohn DV, Forscher BK (1962) Effect of parathyroid extract on the oxidation in vitro of glucose and the production of 14CO-2 by bone and kidney. Biochim Biophys Acta 65:20–26
Esen E et al (2015) PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J Bone Miner Res 30(11):1959–1968
Schmid C, Steiner T, Froesch ER (1982) Parathormone promotes glycogen formation from [14C]glucose in cultured osteoblast-like cells. FEBS Lett 148(1):31–34
Wei J et al (2015) Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell 161(7):1576–1591
Zoidis E, Ghirlanda-Keller C, Schmid C (2011) Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem 348(1–2):33–42
Passi-Even L, Gazit D, Bab I (1993) Ontogenesis of ultrastructural features during osteogenic differentiation in diffusion chamber cultures of marrow cells. J Bone Miner Res 8(5):589–595
Komarova SV, Ataullakhanov FI, Globus RK (2000) Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol 279(4):C1220–C1229
Klein BY et al (1993) Induction of osteoprogenitor cell differentiation in rat marrow stroma increases mitochondrial retention of rhodamine 123 in stromal cells. J Cell Biochem 53(3):190–197
Borle AB, Nichols N, Nichols G Jr (1960) Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem 235:1206–1210
Cohn DV, Forscher BK (1962) Aerobic metabolism of glucose by bone. J Biol Chem 237:615–618
Felix R, Neuman WF, Fleisch H (1978) Aerobic glycolysis in bone: lactic acid production by rat calvaria cells in culture. Am J Physiol 234(1):C51–C55
Neuman WF, Neuman MW, Brommage R (1978) Aerobic glycolysis in bone: lactate production and gradients in calvaria. Am J Physiol 234(1):C41–C50
Guntur AR et al (2014) Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology 155(5):1589–1595
Regan JN et al (2014) Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc Natl Acad Sci USA 111(23):8673–8678
Stegen S et al. (2016) HIF-1alpha promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab 23(2):265–279
Pate KT et al (2014) Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J 33(13):1454–1473
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Owen M, Macpherson S (1963) Cell population kinetics of an osteogenic tissue. II. J Cell Biol 19:33–44
Karner CM et al (2016) Wnt signaling reduces nuclear acetyl-coA levels to suppress gene expression during osteoblast differentiation. J Biol Chem 291(25):13028–13039
DeBerardinis RJ et al (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104(49):19345–19350
Green CR et al (2016) Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol 12(1):15–21
Shiraki N et al (2014) Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab 19(5):780–794
Tonjes M et al (2013) BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med 19(7):901–908
Wang J et al (2009) Dependence of mouse embryonic stem cells on threonine catabolism. Science 325(5939):435–439
Adamson LF, Ingbar SH (1967) Further studies of amino acid transport by embryonic chick bone. J Biol Chem 242(11):2646–2652
Finerman GA, Rosenberg LE (1966) Amino acid transport in bone. Evidence for separate transport systems for neutral amino and imino acids. J Biol Chem 241(7):1487–1493
Hahn TJ, Downing SJ, Phang JM (1969) Amino acid transport in adult diaphyseal bone: contrast with amino acid transport mechanisms in fetal membranous bone. Biochim Biophys Acta 183(1):194–203
Kim SG et al (2006) Differential expression and functional characterization of system L amino acid transporters in human normal osteoblast cells and osteogenic sarcoma cells. Anticancer Res 26(3A):1989–1996
Phang JM, Downing SJ (1973) Amino acid transport in bone: stimulation by cyclic AMP. Am J Physiol 224(1):191–196
Yee JA (1988) Effect of parathyroid hormone on amino acid transport by cultured neonatal mouse calvarial bone cells. J Bone Miner Res 3(2):211–218
Adamson LF, Ingbar SH (1967) Selective alteration by triiodothyronine of amino acid transport in embryonic bone. Endocrinology 81(6):1362–1371
Adamson LF, Ingbar SH (1967) Some properties of the stimulatory effect of thyroid hormones on amino acid transport by embryonic chick bone. Endocrinology 81(6):1372–1378
Baum BJ, Shteyer A (1987) Characteristics of a neutral amino acid transport system (system A) in osteoblastic rat osteosarcoma cells. Exp Cell Res 169(2):453–457
Hahn TJ, Downing SJ, Phang JM (1969) Insulin effect on amino acid transport in bone. Biochim Biophys Acta 184(3):675–677
Hahn TJ, Downing SJ, Phang JM (1971) Insulin effect on amino acid transport in bone: dependence on protein synthesis and Na+. Am J Physiol 220(6):1717–1723
Takarada-Iemata M et al (2011) Glutamate preferentially suppresses osteoblastogenesis than adipogenesis through the cystine/glutamate antiporter in mesenchymal stem cells. J Cell Physiol 226(3):652–665
Uno K et al (2011) A negative correlation between expression profiles of runt-related transcription factor-2 and cystine/glutamate antiporter xCT subunit in ovariectomized mouse bone. J Pharmacol Sci 115(3):309–319
Uno K et al (2011) Negative regulation of osteoblastogenesis through downregulation of runt-related transcription factor-2 in osteoblastic MC3T3-E1 cells with stable overexpression of the cystine/glutamate antiporter xCT subunit. J Cell Physiol 226(11):2953–2964
Harding HP et al (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6(5):1099–1108
Han J et al (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15(5):481–490
Elefteriou F et al (2006) ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab 4(6):441–451
Saito A et al (2011) Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem 286(6):4809–4818
Zhang P et al (2002) The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22(11):3864–3874
Duran RV et al (2012) Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell 47(3):349–358
Huang B et al (2015) mTORC1 prevents preosteoblast differentiation through the notch signaling pathway. PLoS Genet 11(8):e1005426
Jewell JL et al (2015) Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 347(6218):194–198
Nicklin P et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136(3):521–534
Xian L et al (2012) Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 18(7):1095–1101
Zoncu R et al (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334(6056):678–683
Biltz RM et al (1983) Glutamine metabolism in bone. Miner Electrolyte Metab 9(3):125–131
Brown PM, Hutchison JD, Crockett JC (2011) Absence of glutamine supplementation prevents differentiation of murine calvarial osteoblasts to a mineralizing phenotype. Calcif Tissue Int 89(6):472–482
Huang T et al (2016) Aging reduces an ERRalpha-directed mitochondrial glutaminase expression suppressing glutamine anaplerosis and osteogenic differentiation of mesenchymal stem cells. Stem Cells. doi:10.1002/stem.2470.
Masson J et al (2006) Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci 26(17):4660–4671
Niemeier A et al (2008) Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone 43(2):230–237
Catherwood BD et al (1988) Growth of rat osteoblast-like cells in a lipid-enriched culture medium and regulation of function by parathyroid hormone and 1,25-dihydroxyvitamin D. J Bone Miner Res 3(4):431–438
Adamek G et al (1987) Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. Biochem J 248(1):129–137
Chiu KM et al (1999) Carnitine and dehydroepiandrosterone sulfate induce protein synthesis in porcine primary osteoblast-like cells. Calcif Tissue Int 64(6):527–533
Frey JL et al (2015) Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol 35(11):1979–1991
Napoli N et al (2016) Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. doi:10.1038/nrendo.2016.153
Lecka-Czernik B, Rosen CJ (2015) Energy excess, glucose utilization, and skeletal remodeling: new insights. J Bone Miner Res 30(8):1356–1361
Acknowledgements
Original work in the Long lab was supported by NIH grants R01 AR060456 (FL) and F32 AR060674 (CMK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karner, C.M., Long, F. Wnt signaling and cellular metabolism in osteoblasts. Cell. Mol. Life Sci. 74, 1649–1657 (2017). https://doi.org/10.1007/s00018-016-2425-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2425-5