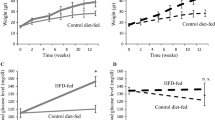
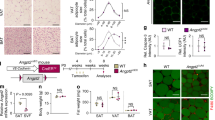
Abstract
Traditionally, the management of diabetes has focused mainly on controlling high blood glucose levels. Unfortunately, despite valiant efforts to normalize this blood glucose, poor medication management predisposes these patients to heart failure. Following diabetes, how the heart utilizes different sources of fuel for energy is key to the development of heart failure. The diabetic heart switches from using both glucose and fats, to predominately using fats as an energy resource for maintaining its activities. This transformation to using fats as an exclusive source of energy is helpful in the initial stages of the disease and is tightly controlled. However, over the progression of diabetes, there is a loss of this controlled supply and use of fats, which ultimately has terrible consequences since the uncontrolled use of fats produces toxic by-products which weaken heart function and cause heart disease. Heparanase is a key player that directs how much fats are provided to the heart and does so in association with several partners like LPL and VEGFs. Together, they regulate the amount of fats supplied, and their subsequent breakdown to provide energy. Following diabetes, there is a disruption in this network resulting in fat oversupply and cell death. Understanding how the heparanase-LPL-VEGFs “ensemble” cooperates, and its dysfunction in the diabetic heart would be useful in restoring metabolic equilibrium and limiting diabetes-related cardiac damage.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Aasum, E., Belke, D. D., Severson, D. L., Riemersma, R. A., Cooper, M., Andreassen, M., & Larsen, T. S. (2002). Cardiac function and metabolism in type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. American Journal of Physiology Heart and Circulatory Physiology, 283(3), H949–H957. https://doi.org/10.1152/ajpheart.00226.2001.
Abboud-Jarrous, G., Atzmon, R., Peretz, T., Palermo, C., Gadea, B. B., Joyce, J. A., & Vlodavsky, I. (2008). Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. The Journal of Biological Chemistry, 283(26), 18167–18176. https://doi.org/10.1074/jbc.M801327200.
Abboud-Jarrous, G., Rangini-Guetta, Z., Aingorn, H., Atzmon, R., Elgavish, S., Peretz, T., & Vlodavsky, I. (2005). Site-directed mutagenesis, proteolytic cleavage, and activation of human proheparanase. Journal of Biological Chemistry, 280(14), 13568–13575. https://doi.org/10.1074/jbc.M413370200.
Abel, E. D. (2004). Glucose transport in the heart. Frontiers in Bioscience: A Journal and Virtual Library, 9, 201–215.
Adameova, A., & Dhalla, N. S. (2014). Role of microangiopathy in diabetic cardiomyopathy. Heart Failure Reviews, 19(1), 25–33. https://doi.org/10.1007/s10741-013-9378-7.
Aerni-Flessner, L., Abi-Jaoude, M., Koenig, A., Payne, M., & Hruz, P. W. (2012). GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle. Cardiovascular Diabetology, 11, 63. https://doi.org/10.1186/1475-2840-11-63.
Allan, C. M., Larsson, M., Jung, R. S., Ploug, M., Bensadoun, A., Beigneux, A. P., Fong, L. G., & Young, S. G. (2017). Mobility of "HSPG-bound" LPL explains how LPL is able to reach GPIHBP1 on capillaries. Journal of Lipid Research, 58(1), 216–225. https://doi.org/10.1194/jlr.M072520.
An, D., Pulinilkunnil, T., Qi, D., Ghosh, S., Abrahani, A., & Rodrigues, B. (2005). The metabolic "switch" AMPK regulates cardiac heparin-releasable lipoprotein lipase. American Journal of Physiology Endocrinology and Metabolism, 288(1), E246–E253. https://doi.org/10.1152/ajpendo.00211.2004.
An, D., & Rodrigues, B. (2006). Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. American Journal of Physiology Heart and Circulatory Physiology, 291(4), H1489–H1506. https://doi.org/10.1152/ajpheart.00278.2006.
Anisimov A, Leppanen VM, Tvorogov D, Zarkada G, Jeltsch M, Holopainen T, Kaijalainen S, Alitalo K (2013) The basis for the distinct biological activities of vascular endothelial growth factor receptor-1 ligands. Science Signaling 6 (282):ra52. doi:https://doi.org/10.1126/scisignal.2003905
Arjunan P, Lin X, Tang Z, Du Y, Kumar A, Liu L, Yin X, Huang L, Chen W, Chen Q, Ye Z, Wang S, Kuang H, Zhou L, Xu K, Chen X, Zeng H, Lu W, Cao Y, Liu Y, Zhao C, Li X (2018) VEGF-B is a potent antioxidant. Proceedings of the National Academy of Sciences of the United States of America 115 (41):10351–10356. doi:https://doi.org/10.1073/pnas.1801379115
Augustus, A. S., Buchanan, J., Park, T. S., Hirata, K., Noh, H. L., Sun, J., Homma, S., D’Armiento, J., Abel, E. D., & Goldberg, I. J. (2006). Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. The Journal of Biological Chemistry, 281(13), 8716–8723. https://doi.org/10.1074/jbc.M509890200.
Azimi-Nezhad, M. (2014). Vascular endothelial growth factor from embryonic status to cardiovascular pathology. Reports of biochemistry & molecular biology, 2(2), 59–69.
Bame, K. J. (2001). Heparanases: Endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology, 11(6), 91R–98R.
Batool, T., Fang, J., Barash, U., Moustakas, A., Vlodavsky, I., & Li, J. P. (2017). Overexpression of heparanase attenuated TGF-beta-stimulated signaling in tumor cells. FEBS Open Bio, 7(3), 405–413. https://doi.org/10.1002/2211-5463.12190.
Belke, D. D., Larsen, T. S., Gibbs, E. M., & Severson, D. L. (2000). Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. American Journal of Physiology Endocrinology and Metabolism, 279(5), E1104–E1113. https://doi.org/10.1152/ajpendo.2000.279.5.E1104.
Borradaile, N. M., & Schaffer, J. E. (2005). Lipotoxicity in the heart. Current Hypertension Reports, 7(6), 412–417.
Boudina, S., & Abel, E. D. (2010). Diabetic cardiomyopathy, causes and effects. Reviews in Endocrine & Metabolic Disorders, 11(1), 31–39. https://doi.org/10.1007/s11154-010-9131-7.
Bry, M., Kivela, R., Leppanen, V. M., & Alitalo, K. (2014). Vascular endothelial growth factor-B in physiology and disease. Physiological Reviews, 94(3), 779–794. https://doi.org/10.1152/physrev.00028.2013.
Bugger, H., & Abel, E. D. (2009). Rodent models of diabetic cardiomyopathy. Disease Models & Mechanisms, 2(9–10), 454–466. https://doi.org/10.1242/dmm.001941.
Bugger, H., Riehle, C., Jaishy, B., Wende, A. R., Tuinei, J., Chen, D., Soto, J., Pires, K. M., Boudina, S., Theobald, H. A., Luptak, I., Wayment, B., Wang, X., Litwin, S. E., Weimer, B. C., & Abel, E. D. (2012). Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. Journal of Molecular and Cellular Cardiology, 52(5), 1019–1026. https://doi.org/10.1016/j.yjmcc.2012.02.001.
Cai, J., Jiang, W. G., Ahmed, A., & Boulton, M. (2006). Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvascular Research, 71(1), 20–31. https://doi.org/10.1016/j.mvr.2005.10.004.
Cai, L., Li, W., Wang, G., Guo, L., Jiang, Y., & Kang, Y. J. (2002). Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes, 51(6), 1938–1948.
Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., Declercq, C., Pawling, J., Moons, L., Collen, D., Risau, W., & Nagy, A. (1996). Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature, 380(6573), 435–439. https://doi.org/10.1038/380435a0.
Chabowski A, Gorski J, Glatz JF, JJ PL, Bonen A (2008) Protein-mediated fatty acid uptake in the heart. Current Cardiology Reviews 4 (1):12–21. doi:https://doi.org/10.2174/157340308783565429
Chiu, A. P., Wan, A., Lal, N., Zhang, D., Wang, F., Vlodavsky, I., Hussein, B., & Rodrigues, B. (2016). Cardiomyocyte VEGF regulates endothelial cell GPIHBP1 to relocate lipoprotein lipase to the coronary lumen during diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology, 36(1), 145–155. https://doi.org/10.1161/ATVBAHA.115.306774.
de Vries, C., Escobedo, J. A., Ueno, H., Houck, K., Ferrara, N., & Williams, L. T. (1992). The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science, 255(5047), 989–991.
Desvergne, B., Michalik, L., & Wahli, W. (2006). Transcriptional regulation of metabolism. Physiological Reviews, 86(2), 465–514. https://doi.org/10.1152/physrev.00025.2005.
Devaux, Y., Vausort, M., Azuaje, F., Vaillant, M., Lair, M. L., Gayat, E., Lassus, J., Ng, L. L., Kelly, D., Wagner, D. R., & Squire, I. B. (2012). Low levels of vascular endothelial growth factor B predict left ventricular remodeling after acute myocardial infarction. Journal of Cardiac Failure, 18(4), 330–337. https://doi.org/10.1016/j.cardfail.2012.01.010.
Diabetes Canada (2018) Diabetes in Canada. Diabetes Canada,
Doolittle, M. H., Ben-Zeev, O., Elovson, J., Martin, D., & Kirchgessner, T. G. (1990). The response of lipoprotein lipase to feeding and fasting. Evidence for posttranslational regulation. The Journal of Biological Chemistry, 265(8), 4570–4577.
Eccles, S. A. (1999). Heparanase: Breaking down barriers in tumors. Nature Medicine, 5(7), 735–736. https://doi.org/10.1038/10455.
Eckel, R. H. (1989). Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. The New England Journal of Medicine, 320(16), 1060–1068. https://doi.org/10.1056/NEJM198904203201607.
Einarson, T. R., Acs, A., Ludwig, C., & Panton, U. H. (2018). Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovascular Diabetology, 17(1), 83. https://doi.org/10.1186/s12933-018-0728-6.
Enerback, S., & Gimble, J. M. (1993). Lipoprotein lipase gene expression: Physiological regulators at the transcriptional and post-transcriptional level. Biochimica et Biophysica Acta, 1169(2), 107–125.
Fairbanks, M. B., Mildner, A. M., Leone, J. W., Cavey, G. S., Mathews, W. R., Drong, R. F., Slightom, J. L., Bienkowski, M. J., Smith, C. W., Bannow, C. A., & Heinrikson, R. L. (1999). Processing of the human heparanase precursor and evidence that the active enzyme is a heterodimer. The Journal of Biological Chemistry, 274(42), 29587–29590.
Fang, Z. Y., Prins, J. B., & Marwick, T. H. (2004). Diabetic cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Endocrine Reviews, 25(4), 543–567. https://doi.org/10.1210/er.2003-0012.
Fantin, A., Vieira, J. M., Plein, A., Denti, L., Fruttiger, M., Pollard, J. W., & Ruhrberg, C. (2013). NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood, 121(12), 2352–2362. https://doi.org/10.1182/blood-2012-05-424713.
Ferrara, N. (2004). Vascular endothelial growth factor: Basic science and clinical progress. Endocrine Reviews, 25(4), 581–611. https://doi.org/10.1210/er.2003-0027.
Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O’Shea, K. S., Powell-Braxton, L., Hillan, K. J., & Moore, M. W. (1996). Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature, 380(6573), 439–442. https://doi.org/10.1038/380439a0.
Ferrara, N., Gerber, H. P., & LeCouter, J. (2003). The biology of VEGF and its receptors. Nature Medicine, 9(6), 669–676. https://doi.org/10.1038/nm0603-669.
Fong, G. H., Rossant, J., Gertsenstein, M., & Breitman, M. L. (1995). Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature, 376(6535), 66–70. https://doi.org/10.1038/376066a0.
Frustaci, A., Kajstura, J., Chimenti, C., Jakoniuk, I., Leri, A., Maseri, A., Nadal-Ginard, B., & Anversa, P. (2000). Myocardial cell death in human diabetes. Circulation Research, 87(12), 1123–1132.
Garfinkel, A. S., Kempner, E. S., Ben-Zeev, O., Nikazy, J., James, S. J., & Schotz, M. C. (1983). Lipoprotein lipase: Size of the functional unit determined by radiation inactivation. Journal of Lipid Research, 24(6), 775–780.
Gingis-Velitski, S., Zetser, A., Kaplan, V., Ben-Zaken, O., Cohen, E., Levy-Adam, F., Bashenko, Y., Flugelman, M. Y., Vlodavsky, I., & Ilan, N. (2004). Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. The Journal of Biological Chemistry, 279(42), 44084–44092. https://doi.org/10.1074/jbc.M402131200.
Glatz, J. F., Bonen, A., Ouwens, D. M., & Luiken, J. J. (2006). Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovascular Drugs and Therapy, 20(6), 471–476. https://doi.org/10.1007/s10557-006-0582-8.
Gleissner, C. A., Galkina, E., Nadler, J. L., & Ley, K. (2007). Mechanisms by which diabetes increases cardiovascular disease. Drug discovery today Disease mechanisms, 4(3), 131–140. https://doi.org/10.1016/j.ddmec.2007.12.005.
Haemmerle, G., Moustafa, T., Woelkart, G., Buttner, S., Schmidt, A., van de Weijer, T., Hesselink, M., Jaeger, D., Kienesberger, P. C., Zierler, K., Schreiber, R., Eichmann, T., Kolb, D., Kotzbeck, P., Schweiger, M., Kumari, M., Eder, S., Schoiswohl, G., Wongsiriroj, N., Pollak, N. M., Radner, F. P., Preiss-Landl, K., Kolbe, T., Rulicke, T., Pieske, B., Trauner, M., Lass, A., Zimmermann, R., Hoefler, G., Cinti, S., Kershaw, E. E., Schrauwen, P., Madeo, F., Mayer, B., & Zechner, R. (2011). ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nature Medicine, 17(9), 1076–1085. https://doi.org/10.1038/nm.2439.
Hagberg, C. E., Falkevall, A., Wang, X., Larsson, E., Huusko, J., Nilsson, I., van Meeteren, L. A., Samen, E., Lu, L., Vanwildemeersch, M., Klar, J., Genove, G., Pietras, K., Stone-Elander, S., Claesson-Welsh, L., Yla-Herttuala, S., Lindahl, P., & Eriksson, U. (2010). Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature, 464(7290), 917–921. https://doi.org/10.1038/nature08945.
Hinkel, R., Howe, A., Renner, S., Ng, J., Lee, S., Klett, K., Kaczmarek, V., Moretti, A., Laugwitz, K. L., Skroblin, P., Mayr, M., Milting, H., Dendorfer, A., Reichart, B., Wolf, E., & Kupatt, C. (2017). Diabetes mellitus-induced microvascular destabilization in the myocardium. Journal of the American College of Cardiology, 69(2), 131–143. https://doi.org/10.1016/j.jacc.2016.10.058.
Huusko, J., Lottonen, L., Merentie, M., Gurzeler, E., Anisimov, A., Miyanohara, A., Alitalo, K., Tavi, P., & Yla-Herttuala, S. (2012). AAV9-mediated VEGF-B gene transfer improves systolic function in progressive left ventricular hypertrophy. Molecular therapy: the journal of the American Society of Gene Therapy, 20(12), 2212–2221. https://doi.org/10.1038/mt.2012.145.
Ibrahimi, A., Bonen, A., Blinn, W. D., Hajri, T., Li, X., Zhong, K., Cameron, R., & Abumrad, N. A. (1999). Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. The Journal of Biological Chemistry, 274(38), 26761–26766.
International Diabetes Federation. (2017). IDF diabetes atlas (8th ed.). Brussels: International Diabetes Federation.
Iozzo, R. V. (2001). Heparan sulfate proteoglycans: Intricate molecules with intriguing functions. The Journal of Clinical Investigation, 108(2), 165–167. https://doi.org/10.1172/JCI13560.
Iozzo, R. V., & San Antonio, J. D. (2001). Heparan sulfate proteoglycans: Heavy hitters in the angiogenesis arena. The Journal of Clinical Investigation, 108(3), 349–355. https://doi.org/10.1172/JCI13738.
Jia, G., DeMarco, V. G., & Sowers, J. R. (2016). Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature Reviews Endocrinology, 12(3), 144–153. https://doi.org/10.1038/nrendo.2015.216.
Jia, G., Hill, M. A., & Sowers, J. R. (2018). Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circulation Research, 122(4), 624–638. https://doi.org/10.1161/CIRCRESAHA.117.311586.
Karar, J., & Maity, A. (2011). PI3K/AKT/mTOR pathway in angiogenesis. Frontiers in Molecular Neuroscience, 4, 51. https://doi.org/10.3389/fnmol.2011.00051.
Karpanen, T., Bry, M., Ollila, H. M., Seppanen-Laakso, T., Liimatta, E., Leskinen, H., Kivela, R., Helkamaa, T., Merentie, M., Jeltsch, M., Paavonen, K., Andersson, L. C., Mervaala, E., Hassinen, I. E., Yla-Herttuala, S., Oresic, M., & Alitalo, K. (2008). Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circulation Research, 103(9), 1018–1026. https://doi.org/10.1161/CIRCRESAHA.108.178459.
Keck, P. J., Hauser, S. D., Krivi, G., Sanzo, K., Warren, T., Feder, J., & Connolly, D. T. (1989). Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science, 246(4935), 1309–1312.
Kessler, G., & Friedman, J. (1998). Metabolism of fatty acids and glucose. Circulation, 98(13), 1351.
Kim, M. S., Wang, Y., & Rodrigues, B. (2012). Lipoprotein lipase mediated fatty acid delivery and its impact in diabetic cardiomyopathy. Biochimica et Biophysica Acta, 1821(5), 800–808. https://doi.org/10.1016/j.bbalip.2011.10.001.
Kivela, R., Bry, M., Robciuc, M. R., Rasanen, M., Taavitsainen, M., Silvola, J. M., Saraste, A., Hulmi, J. J., Anisimov, A., Mayranpaa, M. I., Lindeman, J. H., Eklund, L., Hellberg, S., Hlushchuk, R., Zhuang, Z. W., Simons, M., Djonov, V., Knuuti, J., Mervaala, E., & Alitalo, K. (2014). VEGF-B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in the heart. EMBO Molecular Medicine, 6(3), 307–321. https://doi.org/10.1002/emmm.201303147.
Krilleke, D., DeErkenez, A., Schubert, W., Giri, I., Robinson, G. S., Ng, Y. S., & Shima, D. T. (2007). Molecular mapping and functional characterization of the VEGF164 heparin-binding domain. The Journal of Biological Chemistry, 282(38), 28045–28056. https://doi.org/10.1074/jbc.M700319200.
Kuethe, F., Sigusch, H. H., Bornstein, S. R., Hilbig, K., Kamvissi, V., & Figulla, H. R. (2007). Apoptosis in patients with dilated cardiomyopathy and diabetes: A feature of diabetic cardiomyopathy? Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 39(9), 672–676. https://doi.org/10.1055/s-2007-985823.
Laakso, M. (2011). Heart in diabetes: A microvascular disease. Diabetes Care, 34(Suppl 2), S145–S149. https://doi.org/10.2337/dc11-s209.
Lal, N., Chiu, A. P., Wang, F., Zhang, D., Jia, J., Wan, A., Vlodavsky, I., Hussein, B., & Rodrigues, B. (2017). Loss of VEGFB and its signaling in the diabetic heart is associated with increased cell death signaling. American Journal of Physiology Heart and Circulatory Physiology, 312(6), H1163–H1175. https://doi.org/10.1152/ajpheart.00659.2016.
Lan, L., Wilks, A., Morgan TO, & Di Nicolantonio, R. (1995). Vascular endothelial growth factor: Tissue distribution and size of multiple mRNA splice forms in SHR and WKY. Clinical and experimental pharmacology & physiology Supplement, 22(1), S167–S168.
Lee, J., & Goldberg, I. J. (2007). Lipoproteini lipase-derived fatty acids: Physiology and dysfunction. Current Hypertension Reports, 9(6), 462–466.
Lee, T. Y., Folkman, J., & Javaherian, K. (2010). HSPG-binding peptide corresponding to the exon 6a-encoded domain of VEGF inhibits tumor growth by blocking angiogenesis in murine model. PLoS One, 5(4), e9945. https://doi.org/10.1371/journal.pone.0009945.
Levak-Frank, S., Radner, H., Walsh, A., Stollberger, R., Knipping, G., Hoefler, G., Sattler, W., Weinstock, P. H., Breslow, J. L., & Zechner, R. (1995). Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. The Journal of Clinical Investigation, 96(2), 976–986. https://doi.org/10.1172/JCI118145.
Levy-Adam, F., Feld, S., Cohen-Kaplan, V., Shteingauz, A., Gross, M., Arvatz, G., Naroditsky, I., Ilan, N., Doweck, I., & Vlodavsky, I. (2010). Heparanase 2 interacts with heparan sulfate with high affinity and inhibits heparanase activity. The Journal of Biological Chemistry, 285(36), 28010–28019. https://doi.org/10.1074/jbc.M110.116384.
Li, X., Aase, K., Li, H., von Euler, G., & Eriksson, U. (2001). Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors, 19(1), 49–59.
Li, Y., Zhang, F., Nagai, N., Tang, Z., Zhang, S., Scotney, P., Lennartsson, J., Zhu, C., Qu, Y., Fang, C., Hua, J., Matsuo, O., Fong, G. H., Ding, H., Cao, Y., Becker, K. G., Nash, A., Heldin, C. H., & Li, X. (2008). VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. The Journal of Clinical Investigation, 118(3), 913–923. https://doi.org/10.1172/JCI33673.
Lodish, H. A. B. A., Zipursky, S. L., Matsudaira, P., Baltimore, D., & Darnell, J. (2000). 16.1. Oxidation of glucose and fatty acids to CO2. In Molecular cell biology (4th ed.). New York: W.H. Freeman.
Lopaschuk, G. D., Belke, D. D., Gamble, J., Itoi, T., & Schonekess, B. O. (1994). Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochimica et Biophysica Acta, 1213(3), 263–276.
Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S., & Stanley, W. C. (2010). Myocardial fatty acid metabolism in health and disease. Physiological Reviews, 90(1), 207–258. https://doi.org/10.1152/physrev.00015.2009.
Luiken, J. J., Coort, S. L., Koonen, D. P., van der Horst, D. J., Bonen, A., Zorzano, A., & Glatz, J. F. (2004). Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Archiv: European journal of physiology, 448(1), 1–15. https://doi.org/10.1007/s00424-003-1199-4.
Luiken, J. J., van Nieuwenhoven, F. A., America, G., van der Vusse, G. J., & Glatz, J. F. (1997). Uptake and metabolism of palmitate by isolated cardiac myocytes from adult rats: Involvement of sarcolemmal proteins. Journal of Lipid Research, 38(4), 745–758.
Makinen, T., Olofsson, B., Karpanen, T., Hellman, U., Soker, S., Klagsbrun, M., Eriksson, U., & Alitalo, K. (1999). Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. The Journal of Biological Chemistry, 274(30), 21217–21222.
Mathers, C. D., & Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine, 3(11), e442. https://doi.org/10.1371/journal.pmed.0030442.
McKenzie, E., Tyson, K., Stamps, A., Smith, P., Turner, P., Barry, R., Hircock, M., Patel, S., Barry, E., Stubberfield, C., Terrett, J., & Page, M. (2000). Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochemical and Biophysical Research Communications, 276(3), 1170–1177. https://doi.org/10.1006/bbrc.2000.3586.
Mehlem, A., Palombo, I., Wang, X., Hagberg, C. E., Eriksson, U., & Falkevall, A. (2016). PGC-1alpha coordinates mitochondrial respiratory capacity and muscular fatty acid uptake via regulation of VEGF-B. Diabetes, 65(4), 861–873. https://doi.org/10.2337/db15-1231.
Morigny, P., Houssier, M., Mouisel, E., & Langin, D. (2016). Adipocyte lipolysis and insulin resistance. Biochimie, 125, 259–266. https://doi.org/10.1016/j.biochi.2015.10.024.
Mould, A. W., Greco, S. A., Cahill, M. M., Tonks, I. D., Bellomo, D., Patterson, C., Zournazi, A., Nash, A., Scotney, P., Hayward, N. K., & Kay, G. F. (2005). Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circulation Research, 97(6), e60–e70. https://doi.org/10.1161/01.RES.0000182631.33638.77.
Mysling, S., Kristensen, K. K., Larsson, M., Beigneux, A. P., Gardsvoll, H., Fong, L. G., Bensadouen, A., Jorgensen, T. J., Young, S. G., & Ploug, M. (2016). The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. eLife, 5, e12095. https://doi.org/10.7554/eLife.12095.
Mysling, S., Kristensen, K. K., Larsson, M., Kovrov, O., Bensadouen, A., Jorgensen, T. J., Olivecrona, G., Young, S. G., & Ploug, M. (2016). The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. eLife, 5. https://doi.org/10.7554/eLife.20958.
Niu, Y. G., Hauton, D., & Evans, R. D. (2004). Utilization of triacylglycerol-rich lipoproteins by the working rat heart: Routes of uptake and metabolic fates. The Journal of Physiology, 558(Pt 1), 225–237. https://doi.org/10.1113/jphysiol.2004.061473.
Noh, H. L., Okajima, K., Molkentin, J. D., Homma, S., & Goldberg, I. J. (2006). Acute lipoprotein lipase deletion in adult mice leads to dyslipidemia and cardiac dysfunction. American Journal of Physiology. Endocrinology and Metabolism, 291(4), E755–E760. https://doi.org/10.1152/ajpendo.00111.2006.
Nunn, A. V., Bell, J., & Barter, P. (2007). The integration of lipid-sensing and anti-inflammatory effects: How the PPARs play a role in metabolic balance. Nuclear Receptor, 5(1), 1. https://doi.org/10.1186/1478-1336-5-1.
Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U (1998) Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America 95 (20):11709–11714
Olofsson, B., Pajusola, K., Kaipainen, A., von Euler, G., Joukov, V., Saksela, O., Orpana, A., Pettersson, R. F., Alitalo, K., & Eriksson, U. (1996). Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2576–2581.
Otarod, J. K., & Goldberg, I. J. (2004). Lipoprotein lipase and its role in regulation of plasma lipoproteins and cardiac risk. Current Atherosclerosis Reports, 6(5), 335–342.
Pappachan, J. M., Sebastian, J., Bino, B. C., Jayaprakash, K., Vijayakumar, K., Sujathan, P., & Adinegara, L. A. (2008). Cardiac autonomic neuropathy in diabetes mellitus: Prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgraduate Medical Journal, 84(990), 205–210. https://doi.org/10.1136/pgmj.2007.064048.
Park, J. E., Keller, G. A., & Ferrara, N. (1993). The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Molecular Biology of the Cell, 4(12), 1317–1326.
Pepe, M., Mamdani, M., Zentilin, L., Csiszar, A., Qanud, K., Zacchigna, S., Ungvari, Z., Puligadda, U., Moimas, S., Xu, X., Edwards, J. G., Hintze, T. H., Giacca, M., & Recchia, F. A. (2010). Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circulation Research, 106(12), 1893–1903. https://doi.org/10.1161/CIRCRESAHA.110.220855.
Pikas, D. S., Li, J. P., Vlodavsky, I., & Lindahl, U. (1998). Substrate specificity of heparanases from human hepatoma and platelets. Journal of Biological Chemistry, 273(30), 18770–18777. https://doi.org/10.1074/jbc.273.30.18770.
Pulinilkunnil, T., Abrahani, A., Varghese, J., Chan, N., Tang, I., Ghosh, S., Kulpa, J., Allard, M., Brownsey, R., & Rodrigues, B. (2003). Evidence for rapid "metabolic switching" through lipoprotein lipase occupation of endothelial-binding sites. Journal of Molecular and Cellular Cardiology, 35(9), 1093–1103.
Pulinilkunnil, T., An, D., Yip, P., Chan, N., Qi, D., Ghosh, S., Abrahani, A., & Rodrigues, B. (2004). Palmitoyl lysophosphatidylcholine mediated mobilization of LPL to the coronary luminal surface requires PKC activation. Journal of Molecular and Cellular Cardiology, 37(5), 931–938. https://doi.org/10.1016/j.yjmcc.2004.07.003.
Pulinilkunnil, T., & Rodrigues, B. (2006). Cardiac lipoprotein lipase: Metabolic basis for diabetic heart disease. Cardiovascular Research, 69(2), 329–340. https://doi.org/10.1016/j.cardiores.2005.09.017.
Qi, D., Pulinilkunnil, T., An, D., Ghosh, S., Abrahani, A., Pospisilik, J. A., Brownsey, R., Wambolt, R., Allard, M., & Rodrigues, B. (2004). Single-dose dexamethasone induces whole-body insulin resistance and alters both cardiac fatty acid and carbohydrate metabolism. Diabetes, 53(7), 1790–1797.
Rasanen M, Degerman J, Nissinen TA, Miinalainen I, Kerkela R, Siltanen A, Backman JT, Mervaala E, Hulmi JJ, Kivela R, Alitalo K (2016) VEGF-B gene therapy inhibits doxorubicin-induced cardiotoxicity by endothelial protection. Proceedings of the National Academy of Sciences of the United States of America 113 (46):13144–13149. doi:https://doi.org/10.1073/pnas.1616168113
Robciuc, M. R., Kivela, R., Williams, I. M., de Boer, J. F., van Dijk, T. H., Elamaa, H., Tigistu-Sahle, F., Molotkov, D., Leppanen, V. M., Kakela, R., Eklund, L., Wasserman, D. H., Groen, A. K., & Alitalo, K. (2016). VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metabolism, 23(4), 712–724. https://doi.org/10.1016/j.cmet.2016.03.004.
Rodrigues, B., Cam, M. C., Jian, K., Lim, F., Sambandam, N., & Shepherd, G. (1997). Differential effects of streptozotocin-induced diabetes on cardiac lipoprotein lipase activity. Diabetes, 46(8), 1346–1353.
Rodrigues, B., Cam, M. C., & McNeill, J. H. (1995). Myocardial substrate metabolism: Implications for diabetic cardiomyopathy. Journal of Molecular and Cellular Cardiology, 27(1), 169–179.
Rossetti, L., Giaccari, A., & DeFronzo, R. A. (1990). Glucose toxicity. Diabetes Care, 13(6), 610–630.
Sambandam, N., Abrahani, M. A., St Pierre, E., Al-Atar, O., Cam, M. C., & Rodrigues, B. (1999). Localization of lipoprotein lipase in the diabetic heart: Regulation by acute changes in insulin. Arteriosclerosis, Thrombosis, and Vascular Biology, 19(6), 1526–1534.
Sandesara, P. B., O’Neal, W. T., Kelli, H. M., Samman-Tahhan, A., Hammadah, M., Quyyumi, A. A., & Sperling, L. S. (2018). The prognostic significance of diabetes and microvascular complications in patients with heart failure with preserved ejection fraction. Diabetes Care, 41(1), 150–155. https://doi.org/10.2337/dc17-0755.
Schwenk, R. W., Luiken, J. J., Bonen, A., & Glatz, J. F. (2008). Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovascular Research, 79(2), 249–258. https://doi.org/10.1093/cvr/cvn116.
Senger, D. R., Galli, S. J., Dvorak, A. M., Perruzzi, C. A., Harvey, V. S., & Dvorak, H. F. (1983). Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science, 219(4587), 983–985.
Shibuya, M., & Claesson-Welsh, L. (2006). Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Experimental Cell Research, 312(5), 549–560. https://doi.org/10.1016/j.yexcr.2005.11.012.
Soker, S., Takashima, S., Miao, H. Q., Neufeld, G., & Klagsbrun, M. (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell, 92(6), 735–745.
Sowers, J. R., Epstein, M., & Frohlich, E. D. (2001). Diabetes, hypertension, and cardiovascular disease: An update. Hypertension, 37(4), 1053–1059.
Sun, Y., Jin, K., Childs, J. T., Xie, L., Mao, X. O., & Greenberg, D. A. (2004). Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. Journal of Cerebral Blood Flow and Metabolism, 24(10), 1146–1152. https://doi.org/10.1097/01.WCB.0000134477.38980.38.
Sun, Z., Li, X., Massena, S., Kutschera, S., Padhan, N., Gualandi, L., Sundvold-Gjerstad, V., Gustafsson, K., Choy, W. W., Zang, G., Quach, M., Jansson, L., Phillipson, M., Abid, M. R., Spurkland, A., & Claesson-Welsh, L. (2012). VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. The Journal of Experimental Medicine, 209(7), 1363–1377. https://doi.org/10.1084/jem.20111343.
Talukder, M. A., Kalyanasundaram, A., Zhao, X., Zuo, L., Bhupathy, P., Babu, G. J., Cardounel, A. J., Periasamy, M., & Zweier, J. L. (2007). Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology, 293(4), H2418–H2428. https://doi.org/10.1152/ajpheart.00663.2007.
Teshima, Y., Takahashi, N., Nishio, S., Saito, S., Kondo, H., Fukui, A., Aoki, K., Yufu, K., Nakagawa, M., & Saikawa, T. (2014). Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Circulation Journal: Official Journal of the Japanese Circulation Society, 78(2), 300–306.
Testa, U., Pannitteri, G., & Condorelli, G. L. (2008). Vascular endothelial growth factors in cardiovascular medicine. Journal of Cardiovascular Medicine, 9(12), 1190–1221. https://doi.org/10.2459/JCM.0b013e3283117d37.
van de Weijer, T., Schrauwen-Hinderling, V. B., & Schrauwen, P. (2011). Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovascular Research, 92(1), 10–18. https://doi.org/10.1093/cvr/cvr212.
Vlodavsky, I., Beckhove, P., Lerner, I., Pisano, C., Meirovitz, A., Ilan, N., & Elkin, M. (2012). Significance of heparanase in cancer and inflammation. Cancer microenvironment: official journal of the International Cancer Microenvironment Society, 5(2), 115–132. https://doi.org/10.1007/s12307-011-0082-7.
Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F., & Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death & Disease, 9(2), 119. https://doi.org/10.1038/s41419-017-0135-z.
Vornicova, O., Naroditsky, I., Boyango, I., Shachar, S. S., Mashiach, T., Ilan, N., Vlodavsky, I., & Bar-Sela, G. (2018). Prognostic significance of heparanase expression in primary and metastatic breast carcinoma. Oncotarget, 9(5), 6238–6244. https://doi.org/10.18632/oncotarget.23560.
Waltenberger, J., Claesson-Welsh, L., Siegbahn, A., Shibuya, M., & Heldin, C. H. (1994). Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. The Journal of Biological Chemistry, 269(43), 26988–26995.
Wan, A., & Rodrigues, B. (2016). Endothelial cell-cardiomyocyte crosstalk in diabetic cardiomyopathy. Cardiovascular Research, 111(3), 172–183. https://doi.org/10.1093/cvr/cvw159.
Wang, F., Jia, J., Lal, N., Zhang, D., Chiu, A. P., Wan, A., Vlodavsky, I., Hussein, B., & Rodrigues, B. (2016). High glucose facilitated endothelial heparanase transfer to the cardiomyocyte modifies its cell death signature. Cardiovascular Research, 112(3), 656–668. https://doi.org/10.1093/cvr/cvw211.
Wang, F., Kim, M. S., Puthanveetil, P., Kewalramani, G., Deppe, S., Ghosh, S., Abrahani, A., & Rodrigues, B. (2009). Endothelial heparanase secretion after acute hypoinsulinemia is regulated by glucose and fatty acid. American Journal of Physiology Heart and Circulatory Physiology, 296(4), H1108–H1116. https://doi.org/10.1152/ajpheart.01312.2008.
Wang, F., Wang, Y., Kim, M. S., Puthanveetil, P., Ghosh, S., Luciani, D. S., Johnson, J. D., Abrahani, A., & Rodrigues, B. (2010). Glucose-induced endothelial heparanase secretion requires cortical and stress actin reorganization. Cardiovascular Research, 87(1), 127–136. https://doi.org/10.1093/cvr/cvq051.
Wang, F., Wang, Y., Zhang, D., Puthanveetil, P., Johnson, J. D., & Rodrigues, B. (2012). Fatty acid-induced nuclear translocation of heparanase uncouples glucose metabolism in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(2), 406–414. https://doi.org/10.1161/ATVBAHA.111.240770.
Wang, Y., Puthanveetil, P., Wang, F., Kim, M. S., Abrahani, A., & Rodrigues, B. (2011). Severity of diabetes governs vascular lipoprotein lipase by affecting enzyme dimerization and disassembly. Diabetes, 60(8), 2041–2050. https://doi.org/10.2337/db11-0042.
Wang, Y., Zhang, D., Chiu, A. P., Wan, A., Neumaier, K., Vlodavsky, I., & Rodrigues, B. (2013). Endothelial heparanase regulates heart metabolism by stimulating lipoprotein lipase secretion from cardiomyocytes. Arteriosclerosis, Thrombosis, and Vascular Biology, 33(5), 894–902. https://doi.org/10.1161/ATVBAHA.113.301309.
Weissmann, M., Bhattacharya, U., Feld, S., Hammond, E., Ilan, N., & Vlodavsky, I. (2018). The heparanase inhibitor PG545 is a potent anti-lymphoma drug: Mode of action. Matrix biology: journal of the International Society for Matrix Biology. https://doi.org/10.1016/j.matbio.2018.08.005.
Wende, A. R., & Abel, E. D. (2010). Lipotoxicity in the heart. Biochimica et Biophysica Acta, 1801(3), 311–319. https://doi.org/10.1016/j.bbalip.2009.09.023.
Westermeier, F., Riquelme, J. A., Pavez, M., Garrido, V., Diaz, A., Verdejo, H. E., Castro, P. F., Garcia, L., & Lavandero, S. (2016). New molecular insights of insulin in diabetic cardiomyopathy. Frontiers in Physiology, 7, 125. https://doi.org/10.3389/fphys.2016.00125.
Wilson, A. J., Gill, E. K., Abudalo, R. A., Edgar, K. S., Watson, C. J., & Grieve, D. J. (2018). Reactive oxygen species signalling in the diabetic heart: Emerging prospect for therapeutic targeting. Heart, 104(4), 293–299. https://doi.org/10.1136/heartjnl-2017-311448.
Xu, Y., An, X., Guo, X., Habtetsion, T. G., Wang, Y., Xu, X., Kandala, S., Li, Q., Li, H., Zhang, C., Caldwell, R. B., Fulton, D. J., Su, Y., Hoda, M. N., Zhou, G., Wu, C., & Huo, Y. (2014). Endothelial PFKFB3 plays a critical role in angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(6), 1231–1239. https://doi.org/10.1161/ATVBAHA.113.303041.
Yagyu, H., Chen, G., Yokoyama, M., Hirata, K., Augustus, A., Kako, Y., Seo, T., Hu, Y., Lutz, E. P., Merkel, M., Bensadoun, A., Homma, S., & Goldberg, I. J. (2003). Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. The Journal of Clinical Investigation, 111(3), 419–426. https://doi.org/10.1172/JCI16751.
Yang, L., Zhao, D., Ren, J., & Yang, J. (2015). Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochimica et Biophysica Acta, 1852(2), 209–218. https://doi.org/10.1016/j.bbadis.2014.05.006.
Yeh, W. L., Lin, C. J., & Fu, W. M. (2008). Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Molecular Pharmacology, 73(1), 170–177. https://doi.org/10.1124/mol.107.038851.
Young, M. E., McNulty, P., & Taegtmeyer, H. (2002). Adaptation and maladaptation of the heart in diabetes: Part II: Potential mechanisms. Circulation, 105(15), 1861–1870.
Young, S. G., Davies, B. S., Voss, C. V., Gin, P., Weinstein, M. M., Tontonoz, P., Reue, K., Bensadoun, A., Fong, L. G., & Beigneux, A. P. (2011). GPIHBP1, an endothelial cell transporter for lipoprotein lipase. Journal of Lipid Research, 52(11), 1869–1884. https://doi.org/10.1194/jlr.R018689.
Zcharia, E., Metzger, S., Chajek-Shaul, T., Aingorn, H., Elkin, M., Friedmann, Y., Weinstein, T., Li, J. P., Lindahl, U., & Vlodavsky, I. (2004). Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB journal: official publication of the Federation of American Societies for Experimental Biology, 18(2), 252–263. https://doi.org/10.1096/fj.03-0572com.
Zentilin, L., Puligadda, U., Lionetti, V., Zacchigna, S., Collesi, C., Pattarini, L., Ruozi, G., Camporesi, S., Sinagra, G., Pepe, M., Recchia, F. A., & Giacca, M. (2010). Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. The FASEB Journal, 24(5), 1467–1478. https://doi.org/10.1096/fj.09-143180.
Zhang, D., Wan, A., Chiu, A. P., Wang, Y., Wang, F., Neumaier, K., Lal, N., Bround, M. J., Johnson, J. D., Vlodavsky, I., & Rodrigues, B. (2013). Hyperglycemia-induced secretion of endothelial heparanase stimulates a vascular endothelial growth factor autocrine network in cardiomyocytes that promotes recruitment of lipoprotein lipase. Arteriosclerosis, Thrombosis, and Vascular Biology, 33(12), 2830–2838. https://doi.org/10.1161/ATVBAHA.113.302222.
Zhang, F., Tang, Z., Hou, X., Lennartsson, J., Li, Y., Koch, A. W., Scotney, P., Lee, C., Arjunan, P., Dong, L., Kumar, A., Rissanen, T. T., Wang, B., Nagai, N., Fons, P., Fariss, R., Zhang, Y., Wawrousek, E., Tansey, G., Raber, J., Fong, G. H., Ding, H., Greenberg, D. A., Becker, K. G., Herbert, J. M., Nash, A., Yla-Herttuala, S., Cao, Y., Watts, R. J., & Li, X. (2009). VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 106(15), 6152–6157. https://doi.org/10.1073/pnas.0813061106.
Conflict of Interest
None declared.
Funding
The present study is supported by an operating grant from the Canadian Institutes of Health Research (MOP-133547) and the Heart and Stroke Foundation of Canada (G-16-00014536).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shang, R., Lal, N., Puri, K., Hussein, B., Rodrigues, B. (2020). Involvement of Heparanase in Endothelial Cell-Cardiomyocyte Crosstalk. In: Vlodavsky, I., Sanderson, R., Ilan, N. (eds) Heparanase. Advances in Experimental Medicine and Biology, vol 1221. Springer, Cham. https://doi.org/10.1007/978-3-030-34521-1_30
Download citation
DOI: https://doi.org/10.1007/978-3-030-34521-1_30
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34520-4
Online ISBN: 978-3-030-34521-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)