Abstract
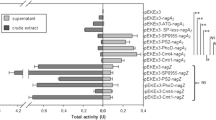
We constructed beta-glucosidase (BGL)-displaying Corynebacterium glutamicum, and direct l-lysine fermentation from cellobiose was demonstrated. After screening active BGLs, Sde1394, which is a BGL from Saccharophagus degradans, was successfully displayed on the C. glutamicum cell surface using porin as an anchor protein, and cellobiose was directly assimilated as a carbon source. The optical density at 600 nm of BGL-displaying C. glutamicum grown on cellobiose as a carbon source reached 23.5 after 48 h of cultivation, which was almost the same as that of glucose after 24 h of cultivation. Finally, Sde1394-displaying C. glutamicum produced 1.08 g/l of l-lysine from 20 g/l of cellobiose after 4 days of cultivation, which was about threefold higher than the amount of produced l-lysine using BGL-secretory C. glutamicum strains (0.38 g/l after 5 days of cultivation). This is the first report on amino acid production using cellobiose as a carbon source by BGL-expressing C. glutamicum.


Similar content being viewed by others
References
Bayan N, Houssin C, Chami M, Leblon G (2003) Mycomembrane and S-layer: two important structures of Corynebacterium glutamicum cell envelope with promising biotechnology applications. J Biotechnol 104:55–56
Bayer EA, Chanzy H, Lamed R, Shoham Y (1998) Cellulose, cellulases and cellulosomes. Curr Opin Struct Biol 8:548–557
Becker J, Wittmann C (2012) Bio-based production of chemicals, materials and fuels—Corynebacterium glutamicum as versatile cell factory. Curr Opin Biotechnol 23:631–640
Gopinath V, Murali A, Dhar KS, Nampoothiri KM (2012) Corynebacterium glutamicum as a potent biocatalyst for the bioconversion of pentose sugars to value-added products. Appl Microbiol Biotechnol 93:95–106
Hansmeier N, Albersmeier A, Tauch A, Damberg T, Ros R, Anselmetti D, Pühler A, Kalinowski J (2006) The surface S-layer gene cspB of Corynebacterium glutamicum is transcriptionally activated by a LuxR-type regulator and located on a 6 kb genomic island absent from the type strain ATCC 13032. Microbiol 152:923–935
Inui M, Kawaguchi H, Murakami S, Vertès AA, Yukawa H (2004) Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J Mol Microbiol Biotechnol 8:243–254
Kind S, Jeong WK, Schröder H, Wittmann C (2010a) Systems-wide metabolic pathway engineering in Corynebacterium glutamicum for bio-based production of diaminopentane. Metab Eng 12:341–351
Kind S, Jeong WK, Schröder H, Wittmann C (2010b) Identification and elimination of the competing N-acetyldiaminopentane pathway for improved production of diaminopentane by Corynebacterium glutamicum. Appl Environ Microbiol 76:5175–5180
Lee SY, Choi JH, Xu Z (2003) Microbial cell-surface display. Trends Biotechnol 21:45–52
Liu J, Barry CE 3rd, Besra GS, Nikaido H (1996) Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J Biol Chem 271:29545–29551
Nakamura N, Yamada R, Kitahara S, Tanaka T, Fukuda H, Kondo A (2008) Effective xylose/cellobiose co-fermentation and ethanol production by xylose-assimilating S. cerevisiae via expression of β-glucosidase on its cell surface. Enzym Microb Technol 43:233–236
Nikaido H, Kim SH, Rosenberg EY (1993) Physical organization of lipids in the cell wall of Mycobacterium chelonae. Mol Microbial 8:1025–1030
Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H (2008a) An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol 81:459–464
Okino S, Suda M, Fujikura K, Inui M, Yukawa H (2008b) Production of d-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 78:449–454
Sakai S, Tsuchida Y, Nakamoto H, Okino S, Ichihashi O, Kawaguchi H, Watanabe T, Inui M, Yukawa H (2007) Effect of lignocellulose-derived inhibitors on growth of and ethanol production by growth-arrested Corynebacterium glutamicum R. Appl Environ Microbiol 73:2349–2353
Sasaki M, Jojima T, Inui M, Yukawa H (2010) Xylitol production by recombinant Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 86:1057–1066
Sasaki M, Jojima T, Inui M, Yukawa H (2008) Simultaneous utilization of d-cellobiose, d-glucose, and d-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 81:691–699
Schneider J, Wendisch VF (2010) Putrescine production by engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 88:859–868
Soual-Hoebeke E, de Sousa-D’Auria C, Chami M, Baucher MF, Guyonvarch A, Bayan N, Salim K, Leblon G (1999) S-layer protein production by Corynebacterium strains is dependent on the carbon source. Microbiol 145:3399–3408
Tanaka T, Kawabata H, Ogino C, Kondo A (2011) Creation of a cellooligosaccharide-assimilating Escherichia coli strain by displaying active beta-glucosidase on the cell surface via a novel anchor protein. Appl Environ Microbiol 77:6265–6270
Tanaka T, Yamada R, Ogino C, Kondo A (2012) Recent developments in yeast cell surface display toward extended applications in biotechnology. Appl Microbiol Biotechnol 95:577–591
Tateno T, Fukuda H, Kondo A (2007a) Production of l-lysine from starch by Corynebacterium glutamicum displaying α-amylase on its cell surface. Appl Microbiol Biotechnol 74:1213–1220
Tateno T, Fukuda H, Kondo A (2007b) Direct production of l-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis α-amylase using cspB promoter and signal sequence. Appl Microbiol Biotechnol 77:533–541
Tateno T, Hatada K, Tanaka T, Fukuda H, Kondo A (2009) Development of novel cell surface display in Corynebacterium glutamicum using porin. Appl Microbiol Biotechnol 84:733–739
Tsuchidate T, Tateno T, Okai N, Tanaka T, Ogino C, Kondo A (2011) Glutamate production from β-glucan using endoglucanase-secreting Corynebacterium glutamicum. Appl Microbiol Biotechnol 90:895–901
Wilson DB (2012) Processive and nonprocessive cellulases for biofuel production—lessons from bacterial genomes and structural analysis. Appl Microbiol Biotechnol 93:497–502
Zuroff TR, Curtis WR (2012) Developing symbiotic consortia for lignocellulosic biofuel production. Appl Microbiol Biotechnol 93:1423–1435
Acknowledgments
This work was supported by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(PDF 57 kb)
Rights and permissions
About this article
Cite this article
Adachi, N., Takahashi, C., Ono-Murota, N. et al. Direct l-lysine production from cellobiose by Corynebacterium glutamicum displaying beta-glucosidase on its cell surface. Appl Microbiol Biotechnol 97, 7165–7172 (2013). https://doi.org/10.1007/s00253-013-5009-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5009-4