Abstract
Background
Mosquito-feeding assays that assess transmission of Plasmodium from man-to-mosquito typically use laboratory mosquito colonies. The microbiome and genetic background of local mosquitoes may be different and influence Plasmodium transmission efficiency. In order to interpret transmission studies to the local epidemiology, it is therefore crucial to understand the relationship between infectivity in laboratory-adapted and local mosquitoes.
Methods
We assessed infectivity of Plasmodium vivax-infected patients from Adama, Ethiopia, using laboratory-adapted (colony) and wild-caught (wild) mosquitoes raised from larval collections in paired feeding experiments. Feeding assays used 4–6 day-old female Anopheles arabiensis mosquitoes after starvation for 12 h (colony) and 18 h (wild). Oocyst development was assessed microscopically 7 days post-feeding. Wild mosquitoes were identified morphologically and confirmed by genotyping. Asexual parasites and gametocytes were quantified in donor blood by microscopy.
Results
In 36 paired experiments (25 P. vivax infections and 11 co-infections with P. falciparum), feeding efficiency was higher in colony (median: 62.5%; interquartile range, IQR: 47.0–79.0%) compared to wild mosquitoes (median: 27.8%; IQR: 17.0–38.0%; Z = 5.02; P < 0.001). Plasmodium vivax from infectious individuals (51.6%, 16/31) infected a median of 55.0% (IQR: 6.7–85.7%; range: 5.5–96.7%; n = 14) of the colony and 52.7% (IQR: 20.0–80.0%; range: 3.2–95.0%; n = 14) of the wild mosquitoes. A strong association (ρ(16) = 0.819; P < 0.001) was observed between the proportion of infected wild and colony mosquitoes. A positive association was detected between microscopically detected gametocytes and the proportion of infected colony (ρ(31) = 0.452; P = 0.011) and wild (ρ(31) = 0.386; P = 0.032) mosquitoes.
Conclusions
Infectivity assessments with colony and wild mosquitoes yielded similar infection results. This finding supports the use of colony mosquitoes for assessments of the infectious reservoir for malaria in this setting whilst acknowledging the importance of mosquito factors influencing sporogonic development of Plasmodium parasites.

Similar content being viewed by others
Background
With the move towards malaria elimination and eradication, new tools and strategies to reduce onward transmission of Plasmodium infections, including transmission-blocking interventions (TBI), are considered highly beneficial [1, 2]. An increasing number of drug- and vaccine-based TBI are in the pipeline [3] and will require monitoring tools for efficacy. Additionally, it is considered highly beneficial to characterize the human infectious reservoir for malaria in low endemic settings approaching elimination, to better target and monitor TBI [4, 5]. Both TBI evaluation and infectious reservoir characterization require robust tools to measure human infectivity to mosquitoes. Mosquito-feeding assays can directly assess Plasmodium transmission from man-to-mosquitoes and play central role to estimate efficacy of TBI and the assessment of the infectious reservoir [6].
Mosquito-feeding assays allow mosquitoes to feed directly on skin of individuals (direct feeding) or on fresh human blood through an artificial membrane (membrane-feeding) [7], after which mosquito midguts are examined for parasite developmental stages (oocysts), the definitive proof that the mosquito became infected [6, 8]. Mosquito-feeding experiments are logistically demanding but increasingly used in field-based studies [5, 9,10,11,12,13,14]. Previous studies used mainly mosquitoes colonized in laboratories for several generations [5, 7, 9, 14,15,16,17,18,19,20,21,22,23]. Laboratory-adapted (colony) mosquitoes offer significant advantage over wild-caught (wild) mosquitoes in terms of logistics, ease of maintenance, flexibility of scaling-up and reproducibility of experiments [24]. However, colony mosquitoes may not fully reflect natural mosquito populations.
Maintenance of insects in artificial breeding conditions favors accumulation of traits that favor survival in the new environment, resulting in a change in genetic make-up over generations [25]. Parasite-mosquito combinations and their susceptibility to malaria infection are regulated at multiple steps during the development of the parasites [26] and numerous factors may modulate this interaction. These factors range from mosquito genetics [27, 28] and immune system [29] to parasite polymorphisms that allow evasion of the mosquito immune system [30]. Environmental factors such as midgut microbiota [31, 32], mosquito larval diet [33, 34], and temperature to support sporogony [35] are also implicated. These findings emphasize that infectivity studies from colony mosquitoes might not represent the infectivity in natural settings and therefore, assessment of the relative permissiveness of colony and wild mosquitoes could assist in the interpretation of mosquito-feeding assays to the local context. In this study, the relative permissiveness to Plasmodium vivax infection of colony and wild Anopheles arabiensis mosquitoes was assessed in paired experiments.
Methods
Study site, immature mosquito stages collection and rearing
Data was collected from September 2018 to February 2019 in Adama, Ethiopia (formerly called Nazareth), a city located within the Great Rift Valley, with an average elevation of ~ 1624 meters above sea level. Extensive irrigation activities characterize the area surrounding Adama with an annual peak malaria transmission season occurring between September and November [5, 36]. Both P. falciparum and P. vivax are endemic; the latter contributes towards ~ 60% of the cases [5, 37].
Immature mosquito stages (larvae/pupae) were collected by standard dipping method from potential breeding sites located at ~ 35 km from the city, close to a hot spring resort (Sodere, 8°24′N, 39°23′E, at an altitude of 1360 meters above sea level) [38]. The breeding habitat is located at a publicly accessible site where there are temporary/permanent puddles made of rock pool or pools in a grassy area (Additional file 1: Figure S1) emanating from a natural hot spring sources which exist throughout the year and form a marshy area. The collected larvae, transported in plastic jars to the field laboratory, were maintained in plastic trays in the original water collected from the breeding sites and provided with fish food (Cichlid Sticks; Tetra, Maidenhead Aquatics, Leicester, UK). Pupae were picked in glass beakers containing sedimented water from the breeding sites and kept in cages until emergence to adults. Adult female An. arabiensis mosquitoes were identified morphologically using standard keys [39, 40]. Colony mosquitoes (> 800th generation) were reared to adulthood as described previously [5]. Mosquitoes of both sources were maintained at the same laboratory settings; developmental stages were reared using fish food (Cichlid Sticks, Tetra) and adult mosquitoes were maintained on sucrose solution (10%) at ambient conditions at temperatures of 26–30 °C and a relative humidity of 60–80% before and after feeding.
Membrane-feeding assays
Venous blood samples (5 ml) were collected after obtaining informed written consent from patients with microscopy-confirmed P. vivax infection attending the Adama Malaria Clinic. Blood collected in lithium heparin tubes (Vacutainer; BD, Oxford, UK) was offered to colony and wild An. arabiensis mosquitoes in parallel using membrane-feeding apparatus as detailed previously [7]. Briefly, 5–6 day-old female mosquitoes were starved for 12 h (colony) and 18 h (wild) before feeding. This timing was decided upon following pilot experiments where aggressiveness was unfavorable for wild mosquitoes after 12 h starvation. We have observed a positive association between starvation time and feeding efficiency (ρ(41) = 0.352; P = 0.024); 18 h was considered appropriate for wild-caught mosquitoes with sufficient numbers of fully fed mosquitoes and minimal mortality. Feeding was performed in the dark for 25 min using water-jacketed glass-feeders (mini-feeder; Coelen Glastechniek, Arnemuiden, the Netherlands) that were covered with an artificial membrane (parafilm) and connected to a circulating water bath (Julabo GmbH; Seelbach, Germany) maintained at 38 °C. Unfed and partially-fed mosquitoes were removed from the holding cages, leaving fully-fed mosquitoes undisturbed. Fully-fed mosquitoes were maintained for 7 days under the same laboratory condition using 10% sucrose solution. At least 10 mosquitoes were dissected, and oocyst presence was assessed microscopically after staining with 1.0% mercurochrome (Sigma-Aldrich, Taufkirchen, Germany). This minimum number was mainly determined by the feeding efficiency and availability of wild mosquitoes. Asexual parasite and gametocyte densities were quantified in thick blood films, screening against 1000 leukocytes.
Mosquito genotyping
A representative set of wild and colony mosquitoes were genotyped using multiplex polymerase chain reaction targeting the intergenic spacer gene of the ribosomal DNA of all cryptic species in the An. gambiae complex as described previously [41], with a few modifications. All conditions, including primers, were as per the original protocol except that the MgCl2 concentration was increased to 2 mM and the amplification time (at 72 °C) was raised to 40 s. Two microliters of eluate of whole mosquito body crushed in phosphate buffer saline was run in a final reaction volume of 25 µl without prior DNA extraction. In every reaction round, negative (non-template and An. stephensi mosquitoes) and positive controls (An. arabiensis colony mosquitoes) were included.
Statistical analysis
All analyses were performed in STATA version 13 (StataCorp., TX, USA) and GraphPad Prism 5.3 (GraphPad Software Inc., CA, USA). Feeding efficiency (proportion of fully-fed mosquitoes) was compared in matched experiments using the Wilcoxon matched-pairs signed-rank test. Proportions were compared by Chi-square and Fischer’s exact tests. Differences between median parasite densities between single-species infections and co-infections were assessed using Wilcoxon rank-sum test. The bias between wild and colony mosquitoes was compared using the Bland-Altman test. The correlation between mosquito infection prevalence and gametocyte density as continuous variable was determined by Spearman’s rank correlation coefficient for colony and wild mosquitoes separately. Logistic regression was performed to compare infection status between colony and wild mosquitoes using individual mosquito data. A fixed effect for human participant was included thus taking into account the number of mosquito experiments and adjusting for correlations between mosquito observations from the same blood donor.
Results
A total of 36 matched membrane-feeding assays (MFA)with colony and wild mosquitoes were performed on blood samples from patients (25 P. vivax single- and 11 mixed-species infections with P. falciparum). The median age of the patients was 23.5 years (interquartile range, IQR: 18.0–29.5) and the majority of participants were male (72.2%, 26/36) (Table 1). A total of 1755 colony and 2303 wild mosquitoes were used for feeding experiments of which 1035 (59.0%) and 662 (28.7%) successfully took a blood meal, respectively. Feeding efficiency varied between colony (median: 62.5%; IQR: 47.0–79.0%) and wild (median: 27.8%; IQR: 17.0–38.0%; Z = 5.02; P < 0.001) mosquitoes (Fig. 1a). Of the total feeding experiments, 52.8% (19/36) infected at least one colony and/or wild mosquitoes. Of P. vivax single-species infected patients, 64.0% (16/25) were infectious to mosquitoes while 27.3% (3/11) of co-infected (P. falciparum + P. vivax) patients infected at least one mosquito (odds ratio, OR: 4.74; 95% confidence interval, CI: 1.0–22.5; P = 0.04). Parasite and gametocyte densities were highest in P. vivax infections (Table 1).
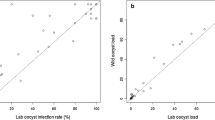
Mosquito infection outcomes in matched colony and wild An. arabiensis membrane-feeding experiments. The proportion of mosquitoes that were fully-fed on the patient blood through the membrane feeder (a) with the resulting proportion of infected mosquitoes (b) are indicated together with the log10-transformed oocyst intensity per midgut of infected mosquito (c) for colony (filled dots) and wild-caught (unfilled dots) mosquitoes. d The association between the proportion of infected mosquitoes (Y-axis) and log10-transformed gametocyte densities/µl (X-axis) measured by microscopy for colony (filled dots) and wild-caught (unfilled dots) mosquitoes. Lines in a–c indicate median values and 25th and 75th percentiles. The asterisks in a indicate a statistically significant difference between the wild and colony mosquitoes (P < 0.001)
After excluding 5 matched experiments for which fewer than 10 wild mosquitoes were available for dissection, there were 31 (21 P. vivax single- and 10 co-infections) successful matched feeding experiments with a minimum of 10 dissected mosquitoes for both feeding approaches. In total, 66.7% (14/21) of P. vivax single-species infected patients infected at least one mosquito. Two patients infected either colony or wild mosquitoes; one of them infecting only colony mosquitoes but not wild (5.8% infected mosquitoes, 4/69) and vice versa (6.7%, 1/15). Infectious individuals infected a median of 55.0% (IQR: 6.7 – 85.7; range: 5.5–96.7%; n = 14) colony and 52.7% (IQR: 20.0–80.0%; range: 3.2–95.0%; n = 14) wild mosquitoes (Fig. 1b). The two infectious co-infected patients infected either of the colony or wild mosquitoes; one infected 5.5% (1/18) colony and the other infected 3.2% (1/32) wild mosquitoes. The median proportions of infected colony and wild mosquitoes were not different between the matched experiments (Z = 0.785; P = 0.433; Fig. 1b). A strong association was observed between the proportion of infected wild and colony mosquitoes (ρ(16) = 0.819; P < 0.001; Fig. 2a). Overall, there was good agreement in the likelihood of becoming infected between wild and colony-reared mosquitoes. Estimation of infectivity to mosquitoes in MFAs showed no significant bias towards either mosquito source (average bias: − 4.79; 95% limits of agreement: − 40.79–31.22; P = 0.381; Fig. 2b). Similarly, there was no difference in the median number of oocysts (Z = 209; P = 0.835; Fig. 1c) detected in infected midguts between the colony (median: 25.0 oocysts/midgut; IQR: 5.0–83.0) and wild (median: 20.5 oocysts/midgut; IQR: 5–47) mosquitoes. In an analysis of individual mosquito data adjusted for human participant, we observed a borderline significant lower proportion of infected wild mosquitoes (OR: 0.67; 95% CI: 0.45–1.00; P = 0.051); plausibly reflecting differences in the number of mosquito observations in experiments.
Comparison of the proportion of infected colony vs wild mosquitoes. a The proportion of infected wild mosquitoes (Y-axis) is plotted against colony mosquitoes (X-axis) for P. vivax single-species infections with at least 10 mosquitoes dissected. The dotted line is the line of perfect agreement. b The differences between the proportion of infected colony and wild mosquitoes plotted against the averages of the two mosquito sources. The average of the proportion of infected colony and wild mosquitoes for each paired infection is indicated in the X-axis vs excess infections in wild mosquitoes (differences between proportions of infected wild mosquitoes vs colony mosquitoes) in the Y-axis. The limits of agreement are indicated as the mean difference (middle dotted line) and the 95% confidence interval of the limit of agreement (mean ± 1.96 SD of differences) with horizontal dotted lines. Unfilled dots indicate P. falciparum + P. vivax co-infections
Microscopically detected parasite density (median: 8765.5; IQR: 2199.5–26158.0; n = 31) was not different between patients with P. vivax single-species infections and patients with co-infections (U(31) = − 103.5; Z = − 0.085; P = 0.933). Gametocytes were more frequently detected by microscopy in patients with P. vivax single-species infections (81.0%, 17/21) than in patients with P. vivax + P. falciparum co- infections (30.0%, 3/10; OR, 9.9; 95% CI, 1.75–56.30; P = 0.010) with a borderline significantly higher gametocyte density among gametocyte-positive P. vivax single-species infections compared to gametocyte-positive co-infections (U(31) = − 149.5; Z = 1.902; P = 0.057). In co-infections, all microscopy-detected gametocytes were P. vivax. Microscopically detectable gametocyte carriers were more infectious than patients without microscopically detectable gametocytes to both colony (65.0%, 13/20 vs 9.1%, 1/11; OR: 18.6; 95% CI: 2.0–176.5; P = 0.002) and wild mosquitoes (60.0%, 12/20 vs 18.2%, 2/11; OR: 6.8; 95% CI: 1.1–39.8; P = 0.021). The proportion of infected colony (ρ(31) = 0.452; P = 0.011) and wild (ρ(31) = 0.386; P = 0.032) mosquitoes associated positively with gametocyte density (Fig. 1d) but not with parasite density assessed by microscopy (ρ(31) = 0.044; P = 0.816) and (ρ(31) = 0.239; P = 0.195), respectively. Morphologically identified wild mosquitoes were confirmed to be An. arabiensis using species-specific PCR for the vast majority of tested mosquitoes (96.5%; 55/57).
Discussion
In recent years, there is increasing interest in transmission assays to evaluate TBI and assess the human infectious reservoir for malaria. More and more laboratories are establishing mosquito colonies to examine infectivity among natural infections [42]. Whilst established colonies offer some advantage in terms of feeding efficiency [43], it is generally assumed that locally relevant mosquitoes are important to allow inference to the local transmission situation. We evaluated the permissiveness of An. arabiensis mosquitoes raised from wild-collected larvae in comparison with colony mosquitoes maintained for over 800 generations in 36 paired MFA. Whilst mosquito feeding rates were markedly higher in colony mosquitoes, we found no evidence for epidemiologically meaningful differences in infection prevalence or infection burden between mosquito sources.
In our experiments, we encountered challenges with the aggressiveness of wild mosquitoes, exemplified by roughly two-fold lower feeding rates on the membrane for wild versus colony mosquitoes, which is not surprising given the selection over several hundred generations in the latter. Colony mosquitoes were maintained using rabbits as source of blood for generations in the present study. Fewer mosquito observations were available for wild mosquitoes on the day of dissection for some of the infections. This may have contributed to the borderline higher proportion of MFA resulting in at least one infected colony mosquito, simply reflecting the higher number of mosquito observations [7]. Despite this, we observed a similar proportion of infected mosquitoes among infectious feeds between colony and wild mosquitoes, in line with several other studies [24, 44]. This holds true when mosquitoes were of the same [24] or different [44] species. The F1 progeny of wild-caught An. funestus compared with colonized An. coluzzii mosquitoes [44] and similarly, colonized An. stephensi mosquitoes compared with their field counterpart raised from wild-caught larvae and pupae [24] were equally susceptible, when the end point was oocyst detection in the midgut.
Importantly, oocyst density was high and similar between the colony and wild mosquitoes in our study in line with previous studies on P. vivax that used mosquitoes of different species [11, 12, 45]. Lower oocyst densities are typically observed in P. falciparum [46,47,48]. Earlier studies also examined sporozoite prevalence and load in feeding experiments; most reporting similar levels between colonized and wild mosquitoes [15]. Similar prevalence but higher sporozoite density (but only at higher sporozoite loads) was detected in the wild mosquitoes in one of the studies [24]. Given the strong association between oocyst prevalence and intensity [49] and the strong association between oocyst density and sporozoite densities [6, 8, 50], it seems intuitive that highly similar oocyst burden, as observed in our study, precludes large differences in sporozoite density. Furthermore, variations in insectary and natural conditions that allow sporogony might potentially explain some of the differences observed [15, 24]. Mosquito innate immune responses can abrogate infections through melanization [51]. We have not observed any evidence for melanization in the present study. In addition, we also examined mosquito guts for pathogens that may influence parasite development such as microsporidia [43] and found no evidence for this. Future studies may nevertheless benefit from examining sporozoites, a limitation of the present study. Investigation of effects of environmental factors on sporogony with a specific focus on midgut microbiota that can influence transmission efficiency by stimulating the mosquito innate immune system and production of metabolites directly impairing parasite survival will also be informative [32]. In addition, mosquito blood-meal size, a poorly studied parameter that may be higher in colony- and membrane-adapted mosquitoes, needs to be considered in future evaluations. We have reared wild collected and colony developmental stages to adults at the same laboratory conditions using the same larval food to minimize the chance this could contribute to a larger body size [52] and subsequently to higher oocyst prevalence and density as a function of larger volume of blood ingested (and therefore more gametocytes) [53, 54]. Future studies would benefit by including wing length measurement as an indication of mosquito body size.
To the best of our knowledge, our findings are the first of its kind with African vivax malaria which is commonly referred to as a major cause of malaria outside sub-Saharan Africa [55]. Ethiopia forms an exception with vivax malaria, contributing towards three-quarters of the global burden together with India and Pakistan [56]. One of the unique features of P. vivax is the earlier generation of gametocytes, i.e. within 3–4 days after the first appearance of asexual parasites [57]. As a result, most patients start infecting mosquitoes before the onset of symptoms [58]. Despite a limited number of studies reporting a lack of association between microscopically determined gametocyte density and infectivity to mosquitoes [59], a very strong association was observed in the likelihood of infectivity between gametocyte densities and both colony and wild mosquitoes in our study. This is concordant with previous reports that used colonized An. dirus [9] and An. arabiensis mosquitoes [5] as well as An. stephensi [60] and An. darligi wild mosquitoes [61] raised from wild-collected immature stages and F1 generations, respectively.
One relevant limitation of our study was the limited sample size, relying on 36 blood donors but a total of 1755 colony and 2303 wild mosquitoes were used for the feeding experiments. We can thus not rule-out subtle differences between colony and wild mosquitoes. It would, however, be questionable whether small differences would render colony mosquitoes less suitable for assessments of the human infectious reservoir or the evaluation of interventions.
Conclusions
The results of the present study indicate that colony mosquitoes perform at a similar level with mosquitoes caught from the wild that reflect the natural phenomenon, indicating colony mosquitoes can be used interchangeably. Our understanding of malaria transmission dynamics would benefit from similar studies in different settings with different vector and parasite species combinations, with a specific focus on mosquito determinants affecting sporogonic development.
Availability of data and materials
Data supporting the conclusions of this article are provided within the article and its additional file. Raw data will be made available upon request.
Abbreviations
- TBI:
-
transmission-blocking interventions
- MFA:
-
membrane feeding assay
References
Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, et al. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406.
Yahiya S, Rueda-Zubiaurre A, Delves MJ, Fuchter MJ, Baum J. The antimalarial screening landscape—looking beyond the asexual blood stage. Curr Opin Chem Biol. 2019;50:1–9.
Delves MJ, Angrisano F, Blagborough AM. Antimalarial transmission-blocking interventions: past, present, and future. Trends Parasitol. 2018;34:735–46.
Stone W, Goncalves BP, Bousema T, Drakeley C. Assessing the infectious reservoir of falciparum malaria: past and future. Trends Parasitol. 2015;31:287–96.
Tadesse FG, Slater HC, Chali W, Teelen K, Lanke K, Belachew M, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. 2018;66:1883–91.
Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, et al. Strong concordance between percent inhibition in oocyst and sporozoite intensities in a Plasmodium falciparum standard membrane-feeding assay. Parasit Vectors. 2019;12:206.
Bousema T, Dinglasan RR, Morlais I, Gouagna LC, van Warmerdam T, Awono-Ambene PH, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE. 2012;7:e42821.
Stone WJ, Eldering M, van Gemert GJ, Lanke KH, Grignard L, van de Vegte-Bolmer MG, et al. The relevance and applicability of oocyst prevalence as a read-out for mosquito feeding assays. Sci Rep. 2013;3:3418.
Kiattibutr K, Roobsoong W, Sriwichai P, Saeseu T, Rachaphaew N, Suansomjit C, et al. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus. Int J Parasitol. 2017;47:163–70.
Ouédraogo AL, Guelbéogo WM, Cohuet A, Morlais I, King JG, Gonçalves BP, et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. Malar World J. 2013;4:16.
Moreno M, Tong-Rios C, Orjuela-Sanchez P, Carrasco-Escobar G, Campo B, Gamboa D, et al. Continuous supply of Plasmodium vivax sporozoites from colonized Anopheles darlingi in the Peruvian Amazon. ACS Infect Dis. 2018;4:541–8.
Zhu G, Xia H, Zhou H, Li J, Lu F, Liu Y, et al. Susceptibility of Anopheles sinensis to Plasmodium vivax in malarial outbreak areas of central China. Parasit Vectors. 2013;6:176.
Bradley J, Stone W, Da DF, Morlais I, Dicko A, Cohuet A, et al. Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. Elife. 2018;7:e34463.
Lin JT, Ubalee R, Lon C, Balasubramanian S, Kuntawunginn W, Rahman R, et al. Microscopic Plasmodium falciparum gametocytemia and infectivity to mosquitoes in Cambodia. J Infect Dis. 2016;213:1491–4.
Moreno M, Tong C, Guzman M, Chuquiyauri R, Llanos-Cuentas A, Rodriguez H, et al. Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am J Trop Med Hyg. 2014;90:612–6.
Thongsahuan S, Baimai V, Junkum A, Saeung A, Min GS, Joshi D, et al. Susceptibility of Anopheles campestris-like and Anopheles barbirostris species complexes to Plasmodium falciparum and Plasmodium vivax in Thailand. Mem Inst Oswaldo Cruz. 2011;106:105–12.
Vallejo AF, Garcia J, Amado-Garavito AB, Arevalo-Herrera M, Herrera S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J. 2016;15:48.
Sattabongkot J, Kumpitak C, Kiattibutr K. Membrane feeding assay to determine the infectiousness of Plasmodium vivax gametocytes. Methods Mol Biol. 2015;1325:93–9.
Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, et al. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin Med J. 1994;107:709–11.
Graves PM. Studies on the use of a membrane feeding technique for infecting Anopheles gambiae with Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1980;74:738–42.
Mulder B, Lensen T, Tchuinkam T, Roeffen W, Verhave JP, Boudin C, et al. Plasmodium falciparum: membrane feeding assays and competition ELISAs for the measurement of transmission reduction in sera from Cameroon. Exp Parasitol. 1999;92:81–6.
Gouagna LC, Okech BA, Kabiru EW, Killeen GF, Obare P, Ombonya S, et al. Infectivity of Plasmodium falciparum gametocytes in patients attending rural health centres in western Kenya. East Afr Med J. 2003;80:627–34.
Ouedraogo AL, Bousema T, Schneider P, de Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS ONE. 2009;4:e8410.
Mohanty AK, Nina PB, Ballav S, Vernekar S, Parkar S, D’souza M, et al. Susceptibility of wild and colonized Anopheles stephensi to Plasmodium vivax infection. Malar J. 2018;17:225.
Ross PA, Hoffmann AA. Rates and patterns of laboratory adaptation in (mostly) insects. J Econ Entomol. 2018;111:501–9.
Alavi Y, Arai M, Mendoza J, Tufet-Bayona M, Sinha R, Fowler K, et al. The dynamics of interactions between Plasmodium and the mosquito: a study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti. Int J Parasitol. 2003;33:933–43.
Harris C, Lambrechts L, Rousset F, Abate L, Nsango SE, Fontenille D, et al. Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum. PLoS Pathogens. 2010;6:e1001112.
Mitri C, Jacques JC, Thiery I, Riehle MM, Xu J, Bischoff E, et al. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathogens. 2009;5:e1000576.
Dimopoulos G. Insect immunity and its implication in mosquito-malaria interactions. Cell Microbiol. 2003;5:3–14.
Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium P47: a key gene for malaria transmission by mosquito vectors. Curr Opin Microbiol. 2017;40:168–74.
Kalappa DM, Subramani PA, Basavanna SK, Ghosh SK, Sundaramurthy V, Uragayala S, et al. Influence of midgut microbiota in Anopheles stephensi on Plasmodium berghei infections. Malar J. 2018;17:385.
Romoli O, Gendrin M. The tripartite interactions between the mosquito, its microbiota and Plasmodium. Parasit Vectors. 2018;11:200.
Okech BA, Gouagna LC, Kabiru EW, Beier JC, Yan G, Githure JI. Influence of age and previous diet of Anopheles gambiae on the infectivity of natural Plasmodium falciparum gametocytes from human volunteers. J Insect Sci. 2004;4:33.
Okech BA, Gouagna LC, Yan G, Githure JI, Beier JC. Larval habitats of Anopheles gambiae s.s. (Diptera: Culicidae) influences vector competence to Plasmodium falciparum parasites. Malar J. 2007;6:50.
Lyons CL, Coetzee M, Terblanche JS, Chown SL. Thermal limits of wild and laboratory strains of two African malaria vector species, Anopheles arabiensis and Anopheles funestus. Malar J. 2012;11:226.
Yohannes M, Petros B. Urban malaria in Nazareth, Ethiopia: parasitological studies. Ethiop Med J. 1996;34:83–91.
File T, Dinka H, Golassa L. A retrospective analysis on the transmission of Plasmodium falciparum and Plasmodium vivax: the case of Adama City, East Shoa Zone, Oromia, Ethiopia. Malar J. 2019;18:193.
Balkew M, Ibrahim M, Koekemoer LL, Brooke BD, Engers H, Aseffa A, et al. Insecticide resistance in Anopheles arabiensis (Diptera: Culicidae) from villages in central, northern and south west Ethiopia and detection of kdr mutation. Parasit Vectors. 2010;3:40.
Gillies M, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–143.
Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Johanesburg: South African Institute for Medical Research; 1968. p. 343.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
The malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission. malERA: an updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017;14:e1002452.
Musiime AK, Okoth J, Conrad M, Ayo D, Onyige I, Rek J, et al. Is that a real oocyst? Insectary establishment and identification of Plasmodium falciparum oocysts in midguts of Anopheles mosquitoes fed on infected human blood in Tororo, Uganda. Malar J. 2019;18:287.
Ndo C, Kopya E, Menze-Djantio B, Toto JC, Awono-Ambene P, Lycett G, et al. High susceptibility of wild Anopheles funestus to infection with natural Plasmodium falciparum gametocytes using membrane feeding assays. Parasit Vectors. 2016;9:341.
Zollner GE, Ponsa N, Garman GW, Poudel S, Bell JA, Sattabongkot J, et al. Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes. Malar J. 2006;5:68.
Gouagna LC, Ferguson HM, Okech BA, Killeen GF, Kabiru EW, Beier JC, et al. Plasmodium falciparum malaria disease manifestations in humans and transmission to Anopheles gambiae: a field study in western Kenya. Parasitology. 2004;128:235–43.
Gouagna LC, Yao F, Yameogo B, Dabiré RK, Ouédraogo J-B. Comparison of field-based xenodiagnosis and direct membrane feeding assays for evaluating host infectiousness to malaria vector Anopheles gambiae. Acta Trop. 2014;130:131–9.
Meibalan E, Barry A, Gibbins MP, Awandu S, Meerstein-Kessel L, Achcar F, et al. P falciparum gametocyte density and infectivity in peripheral blood and skin tissue of naturally infected parasite carriers in Burkina Faso. J Infect Dis. 2019. https://doi.org/10.1093/infdis/jiz680.
Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, et al. Measuring the blockade of malaria transmission - an analysis of the standard membrane feeding assay. Int J Parasitol. 2012;42:1037–44.
Vaughan JA, Noden BH, Beier JC. Sporogonic development of cultured Plasmodium falciparum in six species of laboratory-reared Anopheles mosquitoes. Am J Trop Med Hyg. 1994;51:233–43.
Kumar A, Srivastava P, Sirisena P, Dubey SK, Kumar R, Shrinet J, et al. Mosquito innate immunity. Insects. 2018;9:95.
Araújo Md-S, Gil LHS, e-Silva Ad-A. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar J. 2012;11:261.
Lyimo EO, Koella JC. Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum. Parasitology. 1992;104:233–7.
Akpodiete NO, Diabate A, Tripet F. Effect of water source and feed regime on development and phenotypic quality in Anopheles gambiae (s.l.): prospects for improved mass-rearing techniques towards release programmes. Parasit Vectors. 2019;12:210.
Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106.
Battle KE, Lucas TCD, Nguyen M, Howes RE, Nandi AK, Twohig KA, et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet. 2019;394:332–43.
McKenzie FE, Jeffery GM, Collins WE. Plasmodium vivax blood-stage dynamics. J Parasitol. 2002;88:521–35.
Boyd MF, Kitchen S. On the infectiousness of patients infected with Plasmodium vivax and Plasmodium falciparum. Am J Trop Med Hyg. 1937;17:253–62.
Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology. 1991;102:27–31.
Balabaskaran Nina P, Mohanty AK, Ballav S, Vernekar S, Bhinge S, D’Souza M, et al. Dynamics of Plasmodium vivax sporogony in wild Anopheles stephensi in a malaria-endemic region of western India. Malar J. 2017;16:284.
Bharti AR, Chuquiyauri R, Brouwer KC, Stancil J, Lin J, Llanos-Cuentas A, et al. Experimental infection of the neotropical malaria vector Anopheles darlingi by human patient-derived Plasmodium vivax in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75:610–6.
Acknowledgements
We would like to acknowledge the study participants for their willingness to donate blood samples and the microscopists (Tewabech Lema and Tsehay Orlando) at Adama Malaria Center for their support. We would like to thank the malaria research team at Armauer Hansen Research Institute (Surafel K. Tebeje, Daniel Abebe Mekonnen, Tizita Tsegaye, Tadele Emiru, Tiruwork Fanta and Senya Asfer Sabir) and colleagues from Adama Regional Laboratory (Bayissa Bekele Binagdie, Henok Tadesse Taye and Teshome Bacha) for their continued support. Appreciation also goes to the regional and district health officers for their collaboration. The drivers from AHRI played an important role in making the study successful.
Funding
The study was supported by the Bill and Melinda Gates Foundation grant (INDIE OPP1173572) to FGT, TB and CD. The Armauer Hansen Research Institute (AHRI) has supported WC, TA, EH and EG through its Core funding from Sida and Norad.
Author information
Authors and Affiliations
Contributions
FGT, EG, HM, BP, CD and TB conceived the study, contributed to data analysis and critically commented on the manuscript. FGT, JB and TB analyzed the data. FGT drafted the manuscript. FGT, WC, TA, EH, DY and KL conducted the laboratory study. WC, TA, EH, AG, TT, EE, GS, DY and SWB collected the immature stages (larvae and pupae), reared adult mosquitoes, collected blood samples, performed the feeding experiments, dissected mosquitoes, and analyzed samples. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the National Research Ethical Review Committee (3.10|016\20), Institutional Ethical Review Board of the College of Natural and Computational Sciences of Addis Ababa University, Addis Ababa, Ethiopia, the Observational/Interventions Research Ethics Committee of the London School of Hygiene and Tropical Medicine (15811), London, UK, and AHRI/ALERT Ethics Review Committee, Addis Ababa, Ethiopia (Ref.No.SOM/DRERC/BCH005/2009, PO24/17). All participants provided written informed consent; parent/legal guardians provided consent for participants younger than 18 years.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Larva and/or pupa collection habitats. Breeding habitats were mainly temporary/permanent puddles (a–f) or marshy (g–h) areas, following the streamline of a local hot spring. All the potential breeding habitats were not in use by people living close-by (within a radius of 300–500 m) and had no shading. Larvae were detected at all potential breeding sites with an average larval density of 19.5 larvae per dip. Pupae were detected at 4/9 sites where larvae were detected during a single visit. The median volume of the breeding habitat was 0.20 m3 (IQR: 0.08–0.57 m3; range: 0.004–7.50 m3).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chali, W., Ashine, T., Hailemeskel, E. et al. Comparison of infectivity of Plasmodium vivax to wild-caught and laboratory-adapted (colonized) Anopheles arabiensis mosquitoes in Ethiopia. Parasites Vectors 13, 120 (2020). https://doi.org/10.1186/s13071-020-3998-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-3998-2