Abstract
Background
The colonization of mosquitoes susceptible to Plasmodium vivax via direct membrane feeding assay (DMFA) has the potential to significantly advance our knowledge of P. vivax biology, vector-parasite interaction and transmission-blocking vaccine research. Anopheles darlingi and Anopheles deaneorum are important vectors of malaria in the Western Brazilian Amazon. Since 2018, well-established colonies of these species have been maintained in order to mass produce mosquitoes destined for P. vivax infection. Plasmodium susceptibility was confirmed when the colonies were established, but susceptibility needs to be maintained for these colonies to remain good models for pathogen transmission. Thus, the susceptibility was assessed of colonized mosquitoes to P. vivax isolates circulating in the Western Amazon.
Methods
Laboratory-reared mosquitoes from F10-F25 generations were fed on P. vivax blood isolates via DMFA. Susceptibility was determined by prevalence and intensity of infection as represented by oocyst load seven days after blood feeding, and sporozoite load 14 days after blood feeding. The effect of infection on mosquito survival was evaluated from initial blood feeding until sporogonic development and survival rates were compared between mosquitoes fed on infected and uninfected blood. Correlation was calculated between gametocytaemia and prevalence/intensity of infection, and between oocyst and sporozoite load.
Results
Significant differences were found in prevalence and intensity of infection between species. Anopheles darlingi showed a higher proportion of infected mosquitoes and higher oocyst and sporozoite intensity than An. deaneorum. Survival analysis showed that An. deaneorum survival decreased drastically until 14 days post infection (dpi). Plasmodium vivax infection decreased survival in both species relative to uninfected mosquitoes. No correlation was observed between gametocytaemia and prevalence/intensity of infection, but oocyst and sporozoite load had a moderate to strong correlation.
Conclusions
Colonized An. darlingi make excellent subjects for modelling pathogen transmission. On the other hand, An. deaneorum could serve as a model for immunity studies due the low susceptibility under current colonized conditions. In the application of DMFA, gametocyte density is not a reliable parameter for predicting mosquito infection by P. vivax, but oocyst intensity should be used to schedule sporozoite experiments.
Similar content being viewed by others
Background
Human malaria is an infectious disease caused generally by five protozoa of the Plasmodium genera: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi; although other Plasmodium species, such as Plasmodium simium and Plasmodium cynomolgi, that naturally infected non-human primates have been considered as potential threats to human health through zoonosis [1, 2]. Plasmodium vivax is the dominant malaria parasite in most countries outside of sub-Saharan Africa, and although it is frequently considered to be low pathogenic, it is an important cause of morbidity and mortality in endemic areas in central and South America, and in regions of Asia and Oceania. This species is responsible for the majority of malaria cases in the Brazilian Amazon [3]. The epidemiology of vivax malaria is greatly influenced by hypnozoite formation, which can cause relapses after weeks or months of treatment [4]. Plasmodium vivax is also more efficient at mosquito infection as it is able to maintain the transmission chain at low gametocyte densities [4,5,6]. This P. vivax profile is especially pertinent to malaria control in endemic areas [3, 6]. Plasmodium vivax transmission is currently controlled through vector control and access to effective treatment, but the development of new treatment and control strategies is imperative [7].
However, understanding P. vivax in its asexual and sexual stages has been a great challenge because P. vivax has been available only in non-continuous culture. The colonization of P. vivax-susceptible mosquitoes from endemic areas and the use of standardized direct membrane feeding assay (DMFA) have the potential to rapidly advance our knowledge of P. vivax biology and vector-parasite interaction, and to advance transmission-blocking vaccine (TBV) research [8].
Since 2018, two important vectors of malaria in the Western Brazilian Amazon have been maintained in laboratory colonies: Anopheles darlingi and Anopheles deaneorum [9, 10]. These well-established colonies have been maintained under standardized protocols that allow for the mass production of mosquitoes, and high levels of P. vivax infection. When these colonies were established, both species were tested for susceptibility to P. vivax via DMFA; once susceptibility was confirmed, these colonies were used to study P. vivax-vector interactions [9,10,11]. Biological, vector-parasite interaction and drug assay studies of P. vivax infection were conducted to answer important questions about vivax malaria in the western Amazon [5, 9, 11, 12].
However, it is known that mass rearing and exposure to artificial environments can influence evolutionary changes in the original population, and thereby give rise to strains that are more sensitive to stress and have higher metabolic rates or stronger biosynthetic machinery [13, 14]. Such changes in colonized populations may impact their susceptibility to infection. An evaluation of an An. darlingi colony from Peru showed low to moderate differentiation between field and laboratory populations after 21 generations [15], and wild and colonized Anopheles stephensi mosquitoes were found to be equally susceptible to Plasmodium infection after 66–86 generations [16].
Therefore, the aim to assess whether the mosquito colonies’ susceptibility to P. vivax has remained viable after 25 generations and thus whether the colonies remain good models for vector-parasite studies. To make this assessment, the susceptibility of colonized An. darlingi and An. deaneorum to P. vivax circulating in the Western Amazon was estimated. Susceptibility estimates were made by observing prevalence rates, infection intensity and survival of infected mosquitoes. Additionally, the correlation between gametocytaemia by microscopy and prevalence and infection intensity in these two species was assessed.
Methods
Blood collection from Plasmodium vivax patients
Study participants were recruited from patients diagnosed with vivax malaria by Giemsa-stained blood smears taken at the Centro de Pesquisa em Medicina Tropical (CEPEM), in Porto Velho, Rondonia, a malaria-endemic area in the Amazon region of Brazil.
Malaria transmission in Porto Velho shows seasonal peaks of incidence following the rainy periods (from October to April). In the last five years, Porto Velho has reported increases in malaria cases, with a mean of more than 5000 cases per year. Most cases (~ 95%) are caused by P. vivax [17]. From 2018 to 2020, in all periods of malaria transmission, vivax malaria patients were invited to participate in the study. Volunteers were recruited after fulfillment of the following criteria: thick blood smear positive exclusively for P. vivax, age between 18 and 85 years, absence of signs or symptoms of severe malaria or concomitant disease, with and without a previous history of malaria, non-pregnant and agreement to study procedures. After subjects provided informed consent, about 10 mL of blood was drawn by venipuncture and placed immediately into glass vials coated with heparin to prevent clotting. The tubes were stored in a water flask at 37 °C and transported to the Plataforma de Produção e Infecção de Vetores da Malária (PIVEM) insectary for DMFA. The decision to participate had no effect on malaria treatment and the anti-malarial chemotherapy followed the Brazilian Ministry of Health guidelines.
Plasmodium vivax parasitaemia and gametocyte counts
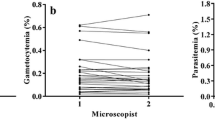
A second thick blood smear was prepared at PIVEM, stained in Giemsa (3% stain working solution), and examined for the presence of malaria parasites under light microscopy using a 100x oil immersion lens. Counts per 200 leukocytes of the sexual (gametocyte) and asexual forms of P. vivax were performed and parasite density was calculated as the number of parasites/ µL by assuming a fixed leukocyte count of 8,000 leukocytes/µL [18]. Results were independently confirmed by two well-trained microscopists and inconsistencies were resolved by a senior microscopist.
Mosquito colonies
Anopheles darlingi and An. deaneorum have been maintained in the insectary of PIVEM in Fiocruz Rondônia since 2018. The mosquitoes were reared at 26 °C ± 1 °C with a relative humidity of 70 ± 10%, and provided with 15% honey [9, 10]. Mosquitoes were fed on parasite-containing blood 3–4 days post-emergence.
Mosquito infection experiments
Prior to DMFA, as described by Moreno et al., female mosquitoes from each colony were deprived of sucrose overnight [19]. A total of 72 P. vivax isolates were used for DMFA, and of these, 17 were used for paired-feeding experiments (An. darlingi vs. An. deaneorum), 43 were used for feeding experiments using just An. darlingi mosquitoes, and 12 were used for feeding experiments using just An. deaneorum mosquitoes. Additional table shows this in more detail in Additional file 1: Table S1.
In each DMFA, cohorts of about 40–100 mosquitoes were fed on P. vivax blood isolates for 30 min. Since the CEPEM clinical site is just 1 km from the PIVEM insectary, it was possible to transport the blood (maintained at 37 °C inside a water flask) and feed the mosquitoes within 5–10 min after blood collection. Two mL of heparinized blood from each volunteer were added to a 5 cm diameter, water-jacketed glass membrane feeder fitted with a slightly stretched Parafilm membrane. Blood was kept at a constant 37 °C during the mosquito feeding. Mosquitoes were allowed to feed for 30 min. After this time, the unfed and partially fed mosquitoes were removed; only fully fed mosquitoes were kept in the experimental cages for subsequent examination of sporogonic development. A cotton wool pad soaked with 15% honey solution was provided regularly and changed every other day until dissection.
The effect of infection on survival was evaluated for mosquitoes fed on infected and uninfected blood, and survival was evaluated from initial blood-feeding until sporogonic development (days 1 to 14).
Mosquito dissections, microscopy and parasite counting
Half of the mosquitoes were dissected on day 7 post blood feeding in order to assess oocyst load in the midgut and the rest were dissected on day 14 post blood feeding in order to assess sporozoite load in the salivary glands (see Additional file 1: Tables S2–S4). Mosquitoes were anaesthetized on ice, placed in a glass beaker (50 mL) with 70% ethanol and then transferred to another glass beaker containing phosphate buffered saline (PBS 1X). The midguts were dissected in PBS 1X, stained with 0.2% commercial mercurochrome (SIGMA), placed under a glass cover, and examined for the presence of oocysts using microscopy (10 X). The salivary glands were dissected in RPMI and transferred to a tube with 15 µL of RPMI in a pool of 2 to 10 salivary glands. Subsequently, the pool was homogenized in a glass tissue grinder and then centrifuged for 30 sec. Sporozoite numbers were counted using a Neubauer chamber hematocytometer under microscope (40X).
Statistics
Statistical analyses were performed using the GraphPad Prism v0.9.0 software. Differences between species in blood-feeding rate and prevalence were analysed using the Chi-square test. The blood-feeding rate was determined by proportion of full engorged mosquitoes after the 30 min to blood feeding and infection prevalence was determined by proportion of mosquitoes infected with oocysts at 7 dpi. Differences in median oocyst and sporozoite production (intensity of infection) were analysed using a Mann-Whitney test that included only individual mosquitoes that produced > 0 oocysts. Spearman’s r was calculated to evaluate the correlation between asexual/gametocytaemia and prevalence, and asexual/gametocytaemia and intensity of infection with a significance level of 0.05. The Kaplan-Meier survival curve was used to represent the probability of survival for mosquitoes fed on infected and uninfected blood over a 14-day follow-up.
Results
A total of 8,427 laboratory-reared mosquitoes were processed: 2,938 mosquitoes (1,477 An. darlingi and 1,461 An. deaneorum) were used for comparative susceptibility (paired feeding) (Table 1), and 1545 mosquitoes (1273 An. darlingi and 272 An. deaneorum) were used for correlation analyses (P. vivax parasitaemia and infection) and survival. A total of 72 P. vivax infections were performed with parasitaemia varying among donors: asexual parasitaemia (180 to 27,405 asexual parasitaemia per µL, median 4200) and gametocytaemia (0 to 1320 gametocytes per µL, median 210) (Table 2). Two infections failed to produce oocysts in both species’ midgut. Data from each patient and number of mosquitoes used to each DMFA are shown in Additional file 1: Table S1.
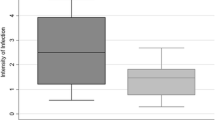
Significant differences were detected in prevalence (p < 0.0001), and oocyst (p = 0.0011) and sporozoite (p < 0.0001) intensity between An. darlingi and An. deaneorum (Fig. 1; Table 1). Anopheles darlingi showed a higher proportion of infected mosquitoes (69.21%), and higher oocyst and sporozoite intensity (median of oocysts = 7.5, range of 1–281; median of sporozoites = 1840, range of 80 − 37,720). On the other hand, An. deaneorum showed a mosquito infection rate of 33.89%, median oocyst production of 4.0 (range of 1–224) and median sporozoite production of 400 (range of 80–13,333) (Fig. 1; Table 1, see Additional file 1: Table S2). The An. darlingi susceptibility was maintained when compared to mosquitoes from the beginning of colonization (prevalence: 70 to 97%) [9], while An. deaneorum showed a decreased on the susceptibility (prevalence: 66 to 100%) [10].
The blood-feeding rate of An. deaneorum was less (59.00%) than that of An. darlingi (86.73%) (Table 1). Mosquito survival was also assessed in comparison to mosquitoes fed with healthy blood. From day 1 (blood feeding) to day 14, mortality was evaluated daily for both species in infected and uninfected groups. Overall, there was a significant difference in survival between infected and uninfected mosquitoes for both species (p < 0.0001). Survival rates were lower among infected mosquitoes (Fig. 2A, B).
Plasmodium vivax infection in Anopheles darlingi (red colour) and Anopheles deaneorum (green colour). A) Distribution of oocyst intensity, each point represents a positive midgut. B) Distribution of sporozoite intensity, each point represents a positive salivary gland. Medians are indicated. Intensity defined by two-sided Mann-Whitney U test. C) Prevalence of infection is shown in the pie charts. Prevalence was defined by two-sided Chi-squared test. Asterisks indicate statistical significance. **P = 0.0011, ****P < 0.0001. The data correspond to 15 independent biological experiments
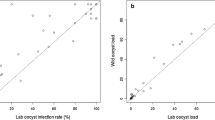
Microscopic examination showed that 92.9% of symptomatic patients had gametocytes circulating in the bloodstream on the day of blood collection. However, no association was found between gametocytaemia and prevalence, or gametocytaemia and infection intensity in either species (Fig. 3A, B, D, E). Blood from symptomatic patients who had no gametocytes circulating in their bloodstreams infected the mosquitoes at rates ranging from 12.5 to 100%. Furthermore, blood from symptomatic patients who had weak gametocytes (between 30 and 200 gametocytes/µL) showed mosquito infection prevalence ranging from 0.0 to 100% (Fig. 3A, D). The correlation analyses between oocysts/mosquito and sporozoites/mosquito revealed a moderate to strong correlation for both species: An. darlingi (r = 0.5430, p value < 0.0001) (Fig. 3C) and An. deaneorum (r = 0.8318, p value < 0.0001) (Fig. 3F). An additional Table shows oocyst and sporozoite numbers of each DMFA (see Additional file 1: Tables S2–S4).
Correlation between gametocytes/µL and prevalence in Anopheles darlingi (A) and Anopheles deaneorum (D). Correlation between gametocytes/µL and oocysts/mosquito in Anopheles darlingi (B) and Anopheles deaneorum (E). Correlation between oocysts/mosquito and sporozoites/mosquito in Anopheles darlingi (C) and Anopheles deaneorum (F). Spearman’s correlation coefficient was used to evaluate the relationship between data
Discussion
Colonies of neotropical mosquito species are being maintained in Brazil to study the parasite-vector relationship [9, 10]. For this purpose, the Plasmodium susceptibility of the colonized mosquitoes needs to be maintained so that the mosquitoes can be used as models for pathogen transmission. At the beginning of colonization, the An. darlingi and An. deaneorum colonies were confirmed to be P. vivax susceptible [9, 10]. Furthermore, when An. darlingi and An. deaneorum susceptibility was compared in first generation mosquitoes, no difference in infection rates and infection intensity was observed [11, 20]. However, the data from generations F10 to F25 show a significant difference in prevalence and infection intensity between An. darlingi and An. deaneorum. Plasmodium vivax prevalence in An. deaneorum was less than 40%, while in An. darlingi was more than 60%. In mosquito Plasmodium susceptibility, it is important consider the interaction among the gut microbiota, the immune system and the parasite. This, because the midgut microbiota has a potential role in mosquito Plasmodium susceptibility [21,22,23]. Although An. darlingi and An. deaneorum have been colonized in the same laboratory conditions, the microbiota of each species may be different and could explain the difference of susceptibility. Studies have demonstrated that different species reared in the same insectary may be host of a different midgut microbiota composition [24, 25]. Investigation to understand the difference in parasite-vector interaction of these two species is necessary.
Another possible explanation for the decreased susceptibility of An. deaneorum is the low blood-feeding rate and the high mortality registered in these An. deaneorum generations. The blood-feeding rate of the An. deaneorum mosquitoes was also less than 60% and their survival drastically decreased after 7 dpi. Although a survival decrease was also observed in the An. darlingi mosquitoes, more than 50% of the infected An. deaneorum mosquitoes were dead at 14 dpi. For efficient parasite transmission, mosquitoes need to survive long enough to allow Plasmodium oocyst development and subsequent salivary gland invasion by sporozoites. The cost of Plasmodium infection to mosquito survival is still a subject of intense debate [26, 27]. The data show a significant difference in survival between infected and uninfected mosquitoes of both species: survival rates were higher among the uninfected. However, this effect could be a relationship with the act of Plasmodium or even in part associated with the act of blood feeding itself. In future, it will be of interest to add unfed mosquitoes to compare with infected and uninfected feeding mosquitoes.
The impact of P. vivax on insect vector survival has been little investigated. Gamage-Mendis et al. showed that P. vivax parasitism did not appear to affect Anopheles tessellatus survival [28], and Andolina et al. found no correlation between Anopheles cracens survival and sporozoite load [29]. However, mosquito survival may be reduced by tissue damage caused during Plasmodium development and migration from the midgut to the salivary glands, and by the activation of a costly immune response [26, 30]. Previous studies found that elevated gametocytaemia (or parasitaemia) may also increase vector mortality [26, 31].
Mosquitoes are infected when they ingest mature gametocytes circulating in the peripheral blood [4]. Therefore, in theory, the density of Plasmodium gametocytes could be used to predict the infection level of Anopheles infected via membrane feeding assay; however, the correlation between P. vivax gametocytaemia and mosquito infections has proven to be variable [32,33,34,35,36,37]. In both An. darlingi and An. deaneorum, no correlation was found between blood gametocyte density and prevalence, or blood gametocyte density and midgut parasite infection load. The data showed that, even with high gametocyte density, the proportion of infected mosquitoes was low in the DMFAs, as well as low gametocyte density showed high infection rate. The data suggest that gametocyte density could not be a good predictor of mosquito infection.
Gametocytes are sometimes undetectable by microscopy [38]. Using real-time reverse transcriptase PCR gametocytaemia, Bharti et al. found a weak correlation between gametocyte density and percentage of mosquitoes infected [34]. There was also a correlation between smear and real-time reverse transcriptase PCR gametocytaemia. Regardless, there is a consensus that gametocyte density is not the only factor affecting the proportion of infected mosquitoes. Parasite genetic variation, human immune response, the maturation and the sex ratio of gametocytes and midgut microbiota composition of vectors, may also be involved, and should be considered [4, 6, 21, 39,40,41]. Future studies are required to better understand P. vivax-mosquito interactions, especially studies that identify what parameters are necessary to improve DMFA. Criteria that predict mosquito infection rates are important because they can help to optimize the mosquito screening process in accord with the experimental specifications used by infection platform.
Positive correlation between the median number of oocysts and sporozoite loads are a criterion that could be used for screening mosquito batches before sporozoite challenge studies. The positive correlation between oocyst level and sporozoite loads has been identified previously in An. tessellatus [28] and Anopheles albimanus [36] infected with P. vivax. Thus, improved screening will allow increase in oocyst production, and consequently, produce more sporozoites.
Currently, there is interest in a whole-parasite vaccine strategy, which requires sporozoite production in live mosquitoes [42]. Vaccine development for vivax malaria is underway and a model to assess vaccine efficacy is urgently needed [36]. One of the aims of PIVEM is to develop a model for assessing potential vaccine candidates. Thus, optimal conditions for mosquito probing and infection are an important requirement for studies that model P. vivax- Anopheles interaction.
Conclusions
This study confirms that An. darlingi colony from the Brazilian Amazon remains highly susceptible to P. vivax infection, and thereby demonstrates that An. darlingi is an excellent species for modelling pathogen transmission. On the other hand, An. deaneorum could serve as a model for immunity studies due the low susceptibility under current colonized conditions. In DMFA, gametocytaemia determined by light microscopy is not a good criterion for predicting mosquito infection by P. vivax, and other predictive factors should be investigated. Finally, the data show that oocyst intensity should be used for scheduling sporozoite experiments.
Availability of data and materials
All relevant data are within the manuscript and its supporting information files.
Abbreviations
- DMFA:
-
Direct Membrane-feeding Assay
- CEPEM:
-
Centro de Pesquisa em Medicina Tropical
- PIVEM:
-
Plataforma de Produção e Infecção de Vetores da Malária
- TBV:
-
Transmission blocking vaccine
References
Brasil P, Zalis MG, Pina-Costa A, Siqueira AM, Júnior CB, Silva S, et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health. 2017;5:e1038–46.
Raja TN, Hu TH, Kadir KA, Mohamad DSA, Rosli N, Wong LL, et al. Naturally acquired human Plasmodium cynomolgi and P. knowlesi infections, Malaysian Borneo. Emerg Infect Dis. 2020;26:1801–9.
WHO. World malaria report. Geneva WH, Organization. 2021. https://doi.org/https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed 22 Mar 2022.
Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–10.
Almeida GG, Costa PAC, Araujo MS, Gomes GR, Carvalho AF, Figueiredo MM, et al. Asymptomatic Plasmodium vivax malaria in the Brazilian Amazon: submicroscopic parasitemic blood infects Nyssorhynchus darlingi. PLoS Negl Trop Dis. 2021;15:e0009077.
Vallejo AF, Garcia J, Amado-Garavito AB, Arevalo-Herrera M, Herrera S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J. 2016;15:48.
Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–66.
Tachibana M, Takashima E, Morita M, Sattabongkot J, Ishino T, Culleton R, et al. Plasmodium vivax transmission-blocking vaccines: progress, challenges and innovation. Parasitol Int. 2021;9:102525.
Araujo SA, Andrade AO, Santos NAC, Pereira DB, Costa GS, Paulo PFM, et al. Brazil’s first free-mating laboratory colony of Nyssorhynchus darlingi. Rev Soc Bras Med Trop. 2019;52:e20190159.
Araujo SA, Santos NAC, Andrade AO, Castro BC, Bastos AS, Resadore F, et al. Description of an automatic copulation induction system used to establish a free-mating laboratory colony of Nyssorhynchus deaneorum from Brazil. Mem Inst Oswaldo Cruz. 2020;115:e200070.
Araujo SA, Andrade AO, Santos NAC, Castro BC, Pereira DB, Rodrigues MMS, et al. First observation of experimental Plasmodium vivax infection of three malaria vectors from the Brazilian Amazon. Vector Borne Zoonotic Dis. 2020;20:517–23.
Penna-Coutinho J, Araujo MS, Aguiar ACC, Sá PM, Rios CT, Medeiros JF, et al. MEFAS, a hybrid of artesunate-mefloquine active against asexual stages of Plasmodium vivax in isolates, inhibits malaria transmission. Int J Parasitol Drugs Resist. 2021;17:150–5.
Aguilar R, Simard F, Kamdem C, Shields T, Glass GE, Garver LS, et al. Genome-wide analysis of transcriptomic divergence between laboratory colony and field Anopheles gambiae mosquitos of the M and S molecular forms. Insect Mol Biol. 2010;19:695–705.
Hoffmann AA, Ross PA. Rates and patterns of laboratory adaptation in (mostly) insects. J Econ Entomol. 2018;111:501–9.
Lainhart W, Bickersmith SA, Moreno M, Tong-Rios C, Vinetz JM, Conn JE. Changes in genetic diversity from field to laboratory during colonization of Anopheles darlingi Root (Diptera: Culicidae). Am J Trop Med Hyg. 2015;93:998–1001.
Mohanty AK, Nina PB, Ballav S, Vernekar S, Parkar S, D’Souza M, et al. Susceptibility of wild and colonized Anopheles stephensi to Plasmodium vivax infection. Malar J. 2018;17:225.
Ministério da Saúde–MS. Dados para o cidadão, Sivep-Malária. https://doi.org/https://public.tableau.com/profile/mal.ria.brasil#!/. Accessed 09 Apr 2022.
WHO. Basic Malaria Microscopy. Geneva, World Health Organization. 2010. https://doi.org/https://apps.who.int/iris/handle/10665/44208. Accessed 09 Apr 2022.
Moreno M, Tong-Rios C, Orjuela-Sanchez P, Carrasco-Escobar G, Campo B, Gamboa D, et al. Continuous supply of Plasmodium vivax sporozoites from colonized Anopheles darlingi in the Peruvian Amazon. ACS Infect Dis. 2018;4:541–8.
Klein TA, Lima JB, Tada MS, Miller R. Comparative susceptibility of anopheline mosquitoes in Rondônia, Brazil to infection by Plasmodium vivax. Am J Trop Med Hyg. 1991;45:463–70.
Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423.
Sharma A, Dhayal D, Singh OP, Adak T, Bhatnagar RK. Gut microbes influence fitness and malaria transmission potential of Asian malaria vector Anopheles stephensi. Acta Trop. 2013;128:41–7.
Gendrin M, Rodgers FH, Yerbanga RS, Ouédraogo JB, Basáñez MG, Cohuet A, et al. Antibiotics in infested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun. 2015;6:5921.
Saab SA, Dohna zu H, Nilsson LKJ, Onorati P, Nakhleh J, Terenius O, et al. The environment and species affect gut bacteria composition in laboratory co-cultured Anopheles gambiae and Aedes albopictus mosquitoes. Sci Rep. 2020;10:3352.
Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Mol Ecol. 2014;23:2727–39.
Ferguson HM, Read AF. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18:256–61.
Martínez-de la Puente J, Gutiérrez-López R, Figuerola J. Do avian malaria reduce longevity. Curr Opin Insect Sci. 2018;28:113–7.
Gamage-Mendis AC, Rajakaruna J, Weerasinghe S, Mendis C, Carter R, Mendis KN. Infectivity of Plasmodium vivax and P. falciparum to Anopheles tessellatus; relationship between oocyst and sporozoite development. Trans R Soc Trop Med Hyg. 1993;87:3–6.
Andolina C, Landier J, Carrara V, Chu CS, Franetich JF, Roth A, et al. The suitability of laboratory bred Anopheles cracens for the production of Plasmodium vivax sporozoites. Malar J. 2015;14:312.
Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–88.
Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Yan J, Soriguer R, Figuerola J. Experimental reduction of host Plasmodium infection load affects mosquito survival. Sci Rep. 2019;9:8782.
Zhu G, Xia H, Zhou H, Li J, Lu F, Liu Y, et al. Susceptibility of Anopheles sinensis to Plasmodium vivax in malarial outbreak areas of central China. Parasit Vectors. 2013;6:176.
Rios-Velásquez CM, Martins-Campos KM, Simões RC, Izzo T, Santos EV, Pessoa AC, et al. Experimental Plasmodium vivax infection of key Anopheles species from the Brazilian Amazon. Malar J. 2013;12:460.
Bharti AR, Chuquiyauri R, Brouwer KC, Stancil J, Lin J, Llanos-Cuentas A, et al. Experimental infection of the neotropical malaria vector Anopheles darlingi by human patient-derived Plasmodium vivax in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75:610–6.
Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N, et al. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol. 2004;41:201–8.
Solarte Y, Manzano MR, Rocha L, Hurtado H, James MA, Arevalo-Herrera M, et al. Plasmodium vivax sporozoite production in Anopheles albimanus mosquitoes for vaccine clinical trials. Am J Trop Med Hyg. 2011;84:28–34.
Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology. 1991;102:27–31.
Beurskens M, Mens P, Schallig, Syafruddin D, Asih PBS, Hermsen R. Quantitative determination of Plasmodium vivax gametocytes by real-time quantitative nucleic acid sequence-based amplification in clinical samples. Am J Trop Med Hyg. 2009;81:366–9.
Reece SE, Drew DR, Gardner A. Sex ratio adjustment and kin discrimination in malaria parasites. Nature. 2008;453:609–14.
Mitri C, Thiery I, Bourgouin C, Paul REL. Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proc Biol Sci. 2009;276:3721–6.
Robert V, Read AF, Essong J, Tchuinkam T, Mulder B, Verhave JP, et al. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans R Soc Trop Med Hyg. 1996;90:621–4.
Minkah NK, Kappe SHI. Malaria vaccine gets a parasite boost in the liver. Nature. 2021;595:173–4.
Acknowledgements
We would like to thank Fiocruz Rondônia for providing support for this study. We are grateful for the assistance provided by the technical, microscopist and medical staff at CEPEM clinics. We also thank the human volunteers for providing the blood samples used in this study.
Funding
This study was funded by CNPq/MS-SCTIE-Decit/Bill & Melinda Gates Fundation N° 23/2019 (grant number 442653/2019-0 and INV-003970) and the International Centers of Excellence for Malaria Research (ICEMR) program GR109237 (CON-80002357). NACS and ASB express their appreciation for a fellowship granted by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (Grant Number 88887.499061/2020-00).
Author information
Authors and Affiliations
Contributions
MSA designed the study; AOA, ASB, MMM and JDCP maintained the mosquito colonies; NACS, AOA, TCS and MSA performed the experiments; MSA, NACS, CBGT and JFM discussed the experiments; MSA and NACS performed the data analysis and drafted the manuscript; LNM and ASF helped with mosquito infection and performed the parasitemia and gametocytemia counts; MSA, CBGT and JFM reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received ethical approval from the CEPEM (protocol number # 28176720.9.0000.0011). Preceding enrolment, the study protocol was explained to all subjects and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Data from each patient and mosquitoes number used to DMFA. Table S2. Oocyst and sporozoites number of each DMFA from paired feeding assay. Table S3. Oocyst and sporozoites number of each DMFA using only Anopheles darlingi. Table S4.Oocyst and sporozoites number of each DMFA using only Anopheles deaneorum.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Santos, N.A.C., Andrade, A.O., Santos, T.C. et al. Evaluation of sustainable susceptibility to Plasmodium vivax infection among colonized Anopheles darlingi and Anopheles deaneorum. Malar J 21, 163 (2022). https://doi.org/10.1186/s12936-022-04204-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04204-8