Abstract
Background
Plant diseases seriously threaten food security, it is urgent to discover efficient and low-risk chemical pesticides. 1,2,4-Oxadiazole derivatives exhibit broad spectrum of agricultural biological activities. For discovering novel molecules with excellent agricultural activities, novel 1,2,4-oxadiazole derivatives were synthesized and evaluated for their agricultural activities.
Result
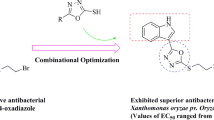
Bioassays results showed that the title compounds exhibited moderate nematocidal activity against Meloidogyne incognita and anti-fungal activity to Rhizoctonia solani. It’s worth noting that compounds 5m, 5r, 5u, 5v, 5x and 5y showed strong antibacterial effects on Xanthomonas oryzae pv. oryzae (Xoo), with EC50 values of 36.25, 24.14, 28.82, 19.44, 25.37 and 28.52 μg/mL, respectively, superior to bismerthiazol (BMT, EC50 = 77.46 μg/mL) and thiodiazole copper (TDC, EC50 = 99.31 μg/mL). Compounds 5p, 5u and 5v exhibited excellent antibacterial ability against Xanthomonas oryzae pv. oryzicola (Xoc), with EC50 values of 31.40, 19.04 and 21.78 μg/mL, respectively, better than that of BMT (EC50 = 68.50 μg/mL) and TDC (EC50 = 91.05 μg/mL). In addition, compound 5v exerted moderate antibacterial effects on rice bacterial leaf blight.
Conclusions
Twenty-six novel 1,2,4-oxadiazole derivatives were obtained and their biological activities were evaluated. Compound 5u and 5v exhibited excellent antibacterial activity Xoo and Xoc. These results indicated that 1,2,4-oxadiazole derivatives containing a trifluoromethyl pyridine moiety could be as potential alternative templates for discovering novel antibacterial agents.

Similar content being viewed by others
Introduction
Crop plants are constantly challenged by a wide variety of pathogens which threaten their growth and survival, such as bacteria, fungus, and plant-parasitic nematodes. As two rice bacterial diseases, rice bacterial leaf blight and rice bacterial leaf streaks caused by Xanthomonas oryzae pv. oryzae (Xoo) and Xanthomonas oryzae pv. oryzicola (Xoc), respectively, serious impact on every stage of plant growth and development. These diseases may result in a loss of up to 80% of the crop and cause severe economic damage [1,2,3,4]. Meanwhile, fungal diseases, for example, rice sheath wilt caused by Rhizoctonia solani, still pose a huge threat to global agriculture [5]. In addition, over 3000 plant species are affected by nematodes worldwide, including ornamental flowers, fruit trees, cereals and vegetables [6,7,8]. The diseases caused by nematodes infecting plants are a serious threat to crop security, causing over $157 billion in economic losses to farmers worldwide [9, 10]. Root-knot nematode is a plant parasitic nematode that affects plant growth by essentially damaging the plant roots [11, 12]. There is an urgent need to devise a method for the effective manual control of these plant diseases, as plants cannot quickly and effectively resist them [13]. Presently, pesticides are often used for agricultural control due to their rapid response to plant diseases [14, 15], however, the long-term abuse of pesticides has led to the emergence of resistance in pathogenic organisms and may pose a risk to human health [16,17,18]. Therefore, developing novel, highly-efficient, and environmentally benign agents against plant diseases remains a daunting task in pesticide sciences.
Heterocyclic structures are widely used in molecular design, and many commodity medicines have been developed [19], such as tioxazafen (Fig. 1), bismerthiazol and fluopyram. As an important five-membered heterocyclic scaffold, 1,2,4-oxadiazoles, with good potent biological properties [20, 21] have been extensively used in pesticide and medicine [22,23,24] molecule design. Moreover, the 1,2,4-oxadiazole heterocycle is a bioisostere of amide but shows better hydrolytic and metabolic stability [22], it is still used as an important pharmacophore to create novel drug molecules. Meanwhile, 1,3,4-thiadiazol and 1,3,4-oxadiazole have been reported to have good biological activities and were used to design drug molecules in pesticide. In our previous work, we designed and synthesized a series of novel 1,3,4-thiadiazol and 1,3,4-oxadiazole derivatives with effective control of bacterial [25, 26], fungal [27,28,29] and plant-parasitic nematodes [30] diseases. In addition, trifluoromethyl pyridine is an important heterocyclic structure containing fluorine, and also a common group in the current commercial pesticides. Fluopyram, containing a trifluoromethyl pyridine moiety, is not only used for control of fungal diseases, but also is used for control plant-parasitic nematodes disease [31, 32].
From the above standpoints, the compounds containing an 1,2,4-oxadiazole heterocycle, 1,3,4-thiadiazol (1,3,4-oxadiazole), or trifluoromethyl pyridine moiety exhibit broad-spectrum agricultural biological activities, which can be used as pharmacophore to design the novel pesticide. Encouraged by these promising results, and in order to obtain compounds with higher biological activity, we employed the structure-based bioisostere strategy, an excellent tool for lead were introduced into 1,2,4-optimisation, 1,3,4-thiadiazol (1,3,4-oxadiazole) and trifluoromethyl pyridine pharmacophores oxadiazole skeleton to design and synthesize a series of novel 1,2,4-oxadiazole derivatives. Meanwhile their agricultural biological activities, including nematocidal, anti-fungal, and antibacterial activity were roundly evaluated. We aimed to discovery novel structure diversity molecules with broad-spectrum activity for development of new pesticides.
Methods
Chemistry
All reagents and chemical materials of the analytically pure were purchased from chemical commercial companies. The reactions were monitored by thin-layer chromatography analysis and the ZF7 ultraviolet analyzer (Yuhua Instrument Co., Ltd. Gong Yi, China). 1H and 13C NMR spectra were obtained on the JEOL-ECX-500 spectrometer (JEOL, Tokyo, Japan) or 400 MHz spectrometer (JEOL, Tokyo, Japan). The melting points of the compounds were measurement by the X-4B melting point instrument of readings were uncorrected (Yidian Physical Optical Instrument Co., Ltd. Shanghai, China). High-resolution mass spectra (ESI TOF (+)) were obtained on the LTQ Orbitrap XL (Thermo Scientific, MO, USA).
General synthesis procedure for compounds 5a–5i
The procedure for synthesizing the target compounds 5a–5i was described in Scheme 1. A mixture of substituted benzonitrile (5.0 mmol), NaOH (3.0 mmol) and hydroxylamine hydrochloride (7.5 mmol) in ethanol/water (30 mL, V:V = 5:1) was refluxed for 4 h. Then, ethanol was removed and the mixture was extracted with ethyl acetate and removed the solvent to obtain intermediate 1. Then chloroacetyl chloride (2.0 mmol) was added to a solution of intermediate 1 (2.0 mmol) in toluene and resulting mixture stirred for 6–8 h at 110–120 °C. After complication of the reaction, the solvent was removed and the residue was recrystallized from ethanol to obtain intermediate 2. Finally, K2CO3 (1.0 mmol) was added to a solution of corresponding 1,3,4-oxadiazole/thiadiazole thiol intermediate (1.0 mmol) in MeCN (20 mL) and stirred at room temperature for 0.5 h. Then intermediate 2 (1.0 mmol) was added and the mixture was refluxed. After complication of the reaction, the solvent was removed and the residue was recrystallized from ethanol to obtain the target compounds 5a–5i with 34.8–62.3% yields.
General synthesis procedure for compounds 5j–5r
A mixture of 2,3-dichloro-5-(trifluoromethyl) pyridine (5.0 mmol) and 4-hydroxybenzonitrile (5.0 mmol) in DMF (8 mL) was first stirred at room temperature for 0.5 h. Then K2CO3 (10.0 mmol) was added and the mixture was refluxed for 8 h. After complication of the reaction, the mixture was poured into 100 mL of ethanol. The precipitate was filtered off and to obtain intermediate 3. Then, a mixture of intermediate 3 (3.0 mmol), NaHCO3 (3.0 mmol) and hydroxylamine hydrochloride (4.5 mmol) in ethanol (20 mL) was refluxed for 2 h. The solvent was removed and the residue was poured into water. The mixture was extracted with ethyl acetate and removed the solvent to obtain intermediate 4. Then substituted acyl chloride (2.0 mmol) was added to a solution of intermediate 4 (2.0 mmol) in toluene and resulting mixture stirred for 6–8 h at 110–120 °C. After complication of the reaction, the solvent was removed and the residue was recrystallized from ethanol to afford the target compounds 5j–5r with 31.7–62.9% yields (Scheme 2).
General synthesis procedure for compounds 5s–5z
K2CO3 (1.0 mmol) was added to a solution of substituted phenol (1.0 mmol) in MeCN (20 mL) and stirred at room temperature for 0.5 h. Then compound 5r (1.0 mmol) was added and the mixture was refluxed for 4–6 h. After complication of the reaction, the solvent was removed and the residue was recrystallized from ethanol to obtain the target compounds 5s–5z with 41.8–61.8% yields (Scheme 3).
5-(((5-(4-fluorobenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-3-phenyl-1,2,4-oxadiazole (5a) Yellow solid; yield 55.0%; mp: 106.5–107.1 °C; 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.6 Hz, 3H, Ph-H), 7.47 (d, J = 7.6 Hz, 3H, Ph-H) 7.19 (d, J = 8.6 Hz, 2H, Ph-H), 4.75 (s, 2H, –CH2–). 13C NMR (101 MHz, CDCl3) δ 174.47, 168.81, 165.77, 162.59, 161.69, 131.52, 129.33, 129.33, 128.77, 128.77, 127.49, 127.49, 126.13, 119.59, 116.62, 116.40, 31.44; HRMS (ESI) calcd for C17H12N4O2SF [M+H]+: 355.06534, found 355.06595.
5-(((5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-3-(4-fluorophenyl)-1,2,4-oxadiazole (5b) Yellow solid; yield 45.0%; mp: 101.9–103.2 °C; 1H NMR (400 MHz, CDCl3) δ 8.05 (dd, J = 8.9, 5.4 Hz, 2H, Ph-H), 7.94 (d, J = 8.7 Hz, 2H, Ph-H), 7.48 (d, J = 8.7 Hz, 2H, Ph-H), 7.15 (t, J = 8.7 Hz, 2H, Ph-H), 4.75 (s, 2H, –CH2–). 13C NMR (101 MHz, CDCl3) δ 174.96, 169.70, 168.77, 162.58 161.73, 131.42, 129.57, 129.57, 129.29, 129.29, 128.89, 128.89, 127.86, 127.86, 127.54, 126.32, 31.46; HRMS (ESI) calcd for C17H11N4O2SFCl [M+H]+: 389.02698, found 353.02681.
5-(((5-(4-chlorobenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-3-phenyl-1,2,4-oxadiazole (5c) Brown solid; yield 57.3%; mp: 83.5–84.7 °C; 1H NMR (400 MHz, CDCl3) δ 8.06–8.02 (m, 2H, Ph-H), 7.51–7.45 (m, 3H, Ph-H), 7.32–7.27 (m, 2H, Ph-H), 7.22 (t, J = 6.8 Hz, 2H, Ph-H), 4.78 (s, 2H, –CH2–), 4.35 (s, 2H, –CH2–). 13C NMR (101 MHz, CDCl3) δ 174.85, 170.43, 168.70, 162.95, 134.90, 133.67, 131.43, 130.15, 130.15, 129.25, 129.25, 128.88, 128.88, 127.48, 127.48, 126.27, 35.80, 27.65; HRMS (ESI) calcd for C18H14N4OS2Cl [M+H]+: 401.02856, found 401.02921.
5-(((5-(4-fluorophenyl)-1,3,4-thiadiazol-2-yl)thio)methyl)-3-(o-tolyl)-1,2,4-oxadiazole (5d) Yellow solid; yield 48.6%; mp: 98.1–98.7 °C; 1H NMR (500 MHz, CDCl3) δ 7.97–7.92 (m, 3H, Ph-H), 7.47 (d, J = 8.7 Hz, 2H, Ph-H), 7.40–7.36 (m, 1H, Ph-H), 7.32–7.26 (m, 2H, Ph-H), 4.76 (s, 2H, –CH2–), 2.59 (s, 3H, –CH3). 13C NMR (126 MHz, CDCl3) δ 173.38, 169.47, 165.86, 162.06, 138.42, 131.54, 130.97, 130.22, 130.22, 129.63, 129.63, 128.16, 126.10, 125.47, 121.83, 114.02, 26.85, 22.28; HRMS (ESI) calcd for C18H15N4OS2 [M+H]+: 367.06766, found 367.06818.
5-(((5-(3-chlorobenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-3-(o-tolyl)-1,2,4-oxadiazole (5e) Yellow solid; yield 62.3%; mp: 82.6–83.2 °C; 1H NMR (500 MHz, CDCl3) δ 7.94–7.91 (m, 1H, Ph-H), 7.39 (td, J = 7.6, 1.4 Hz, 1H, Ph-H), 7.32–7.26 (m, 4H, Ph-H), 7.23–7.19 (m, 2H, Ph-H), 4.67 (s, 2H, –CH2–), 4.15 (s, 2H, –CH2–), 2.58 (s, 3H, –CH3). 13C NMR (126 MHz, CDCl3) δ 173.34, 169.40, 166.83, 162.52, 138.43, 133.84, 131.76, 131.55, 131.55, 130.97, 130.24, 130.24, 129.25, 129.25, 126.12, 125.47, 31.35, 26.69, 22.28; HRMS (ESI) calcd for C19H16N4O2SCl [M+H]+: 399.06714, found 399.06770.
3-(4-chlorophenyl)-5-(((5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-1,2,4-oxadiazole (5f) White solid; yield 60.2%; mp: 106.6–108.1 °C; 1H NMR (500 MHz, CDCl3) δ 8.03–7.97 (m, 4H, Ph-H), 7.46–7.42 (m, 2H, Ph-H), 7.21–7.16 (m, 2H, Ph-H), 4.74 (s, 2H, –CH2–). 13C NMR (126 MHz, CDCl3) δ 174.75, 168.15, 165.95, 161.70, 137.79, 130.03, 130.03, 129.59, 129.59, 129.01, 129.01, 128.91, 128.91, 124.74, 119.65, 116.72, 26.83; HRMS (ESI) calcd for C17H11N4O2SFCl [M+H] + : 389.02637, found 389.02698.
5-(((5-(4-chlorobenzyl)-1,3,4-oxadiazol-2-yl)thio)methyl)-3-(4-chlorophenyl)-1,2,4-oxadiazole (5 g) Yellow solid; yield 56.3%; mp: 84.7–86.0 °C; 1H NMR (500 MHz, CDCl3) δ 7.99–7.95 (m, 2H, Ph-H) 7.46–7.43 (m, 2H, Ph-H), 7.32–7.28 (m, 2H, Ph-H), 7.22–7.19 (m, 2H, Ph-H), 4.77 (s, 2H, –CH2–), 4.34 (s, 2H, –CH2–). 13C NMR (126 MHz, CDCl3) δ 175.22, 170.54, 168.02, 162.96, 137.69, 134.97, 133.80, 130.26, 130.26, 129.59, 129.59, 129.14, 129.14, 128.89, 128.89, 124.88, 35.90, 27.63; HRMS (ESI) calcd for C18H13N4O2SCl2 [M+H]+: 419.01233, found 419.01308.
5-(((5-(4-chlorobenzyl)-1,3,4-thiadiazol-2-yl)thio)methyl)-3-(4-chlorophenyl)-1,2,4-oxadiazole (5h) Yellow solid; yield 34.8%; mp: 83.0–84.3 °C; 1H NMR (500 MHz, CDCl3) δ 7.99–7.93 (m, 2H, Ph-H), 7.48–7.40 (m, 2H, Ph-H), 7.31–7.26 (m, 2H, Ph-H), 7.21 (d, J = 8.5 Hz, 2H, Ph-H), 4.65 (s, 2H, –CH2–), 4.15 (s, 2H, -CH2-). 13C NMR (126 MHz, CDCl3) δ 174.67, 168.08, 166.88, 162.41, 137.80, 133.87, 131.71, 130.39, 130.39, 129.61, 129.61, 129.30, 129.30, 129.00, 128.77, 124.71, 31.44, 26.67; HRMS (ESI) calcd for C18H13N4OS2Cl2 [M+H]+: 434.98953, found 434.99023.
5-(((5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)thio)methyl)-3-(p-tolyl)-1,2,4-oxadiazole (5i) Yellow solid; yield 41.5%; mp: 106.7–107.8 °C; 1H NMR (400 MHz, CDCl3) δ 7.97–7.92 (m, 4H, Ph-H), 7.47 (d, J = 8.6 Hz, 2H, Ph-H), 7.27 (d, J = 8.2 Hz, 2H, Ph-H), 4.74 (s, 2H, –CH2–), 2.41 (s, 3H, –CH3). 13C NMR (101 MHz, CDCl3) δ 174.16, 165.79, 161.95, 141.94, 138.31, 133.80, 129.52, 129.52, 129.59, 129.59, 128.00, 128.00, 127.44, 127.44, 123.32 121.75, 26.82, 21.62; HRMS (ESI) calcd for C18H15N4OS2 [M+H]+: 367.06763, found 367.06818.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-phenyl-1,2,4-oxadiazole (5j) White solid; yield 31.7%; mp: 94.5–95.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.63 (d, J = 2.2 Hz, 1H, Pyridine-H), 8.56 (dd, J = 2.1, 0.9 Hz, 1H, Pyridine-H), 8.23–8.17 (m, 4H, Ph-H), 7.79–7.74 (m, 1H, Ph-H), 7.71–7.66 (m, 2H, Ph-H), 7.52–7.48 (m, 2H, Ph-H). 13C NMR (101 MHz, DMSO-d6) δ 175.99, 168.17, 160.92, 155.64, 143.64, 137.85, 133.92, 130.09, 130.09, 129.36, 129.36, 128.43, 124.79, 124.06, 123.81, 123.09, 123.09, 122.52, 122.14, 119.16; HRMS (ESI) calcd for C20H12N3O2F3Cl [M+H]+: 418.05539, found 418.05647.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-(4-fluorophenyl)-1,2,4-oxadiazole (5k) White solid; yield 41.8%; mp: 106.5–107.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.64 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.56 (dd, J = 2.1, 0.9 Hz, 1H, Pyridine-H), 8.23–8.17 (m, 4H, Ph-H), 7.79–7.74 (m, 1H, Ph-H), 7.78–7.74 (m, 2H, Ph-H), 7.54–7.47 (m, 2H, Ph-H). 13C NMR (101 MHz, DMSO-d6) δ 175.15, 168.22, 160.91, 155.69, 143.62, 138.76, 137.89, 130.28, 130.28, 129.37, 129.37, 124.79, 124.05, 123.91, 123.11, 123.11, 122.60, 122.19 119.16, 119.16; HRMS (ESI) calcd for C20H11N3O2F4Cl [M+H]+: 436.04312, found 436.04704.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-(4-methoxyphenyl)-1,2,4-oxadiazole (5l) Yellow solid; yield 62.9%; mp: 95.2–96.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.69 (d, J = 2.0 Hz, 1H, Pyridine-H), 8.61 (d, J = 1.1 Hz, 1H, Pyridine-H), 8.25–8.19 (m, 4H, Ph-H), 7.57–7.53 (m, 2H, Ph-H), 7.27 (d, J = 8.9 Hz, 2H, Ph-H), 3.95 (s, 3H, –OCH3). 13C NMR (101 MHz, DMSO-d6) δ 175.83, 167.99, 160.94, 155.53, 143.62, 137.88, 130.47, 130.47, 129.31, 129.31, 124.79, 124.21, 123.07, 123.07, 122.48, 122.15, 119.12, 116.11, 115.51, 115.51, 56.49; HRMS(ESI) calcd for C21H14N3O3F3Cl; [M+H]+: 448.06703, found 448.06580.
(E)-3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-styryl-1,2,4-oxadiazole (5 m) Light yellow solid; yield 41.7%; mp: 121.0–121.5 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.64 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.56 (dd, J = 2.1, 1.0 Hz, 1H, Pyridine-H), 8.14–8.09 (m, 2H, –CH–), 7.98 (d, J = 16.4 Hz, 1H, Ph-H), 7.90–7.86 (m, 2H Ph-H), 7.52–7.46 (m, 6H, Ph-H). 13C NMR (101 MHz, DMSO-d6) δ 175.99, 167.89, 160.93, 155.54, 144.29, 143.34, 137.88, 134.69, 131.20, 129.51, 129.51, 129.26, 129.26, 128.92, 124.79, 124.19, 123.07, 123.07, 122.48, 122.12, 119.14, 110.66; HRMS (ESI) calcd for C22H14N3O2F3Cl [M+H]+: 444.07135, found 444.07212.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-(4-nitrophenyl)-1,2,4-oxadiazole (5n) White solid; yield 35.8%; mp: 182.4–183.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.64 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.65 (d, J = 1.9 Hz, 1H, Pyridine-H), 8.49 (d, J = 3.4 Hz, 4H, Ph-H), 8.24–8.19 (m, 2H, Ph-H), 7.56–7.50 (m, 2H, Ph-H). 13C NMR (101 MHz, DMSO-d6) δ 175.1, 168.14, 160.61, 157.93, 155.62, 143.61, 137.82, 132.66, 131.32, 129.75, 129.33, 129.33, 124.78, 123.90, 123.90, 122.50, 121.84, 120.51, 119.14, 117.25; HRMS (ESI) calcd for C20H11N4O4F3Cl [M+H]+: 463.04154, found 463.04025.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-(4-(trifluoromethyl)phenyl)-1,2,4-oxadiazole (5o) White solid; yield 44.3%; mp: 102.8–103.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.59 (d, J = 2.2 Hz, 1H, Pyridine-H), 8.51 (d, J = 1.0 Hz, 1H, Pyridine-H), 8.36 (d, J = 8.3 Hz, 2H, Pyridine-H) 8.17–8.13 (m, 2H, Ph-H), 8.00 (d, J = 8.4 Hz, 2H, Ph-H), 7.48–7.45 (m, 2H, Ph-H). 13C NMR (101 MHz, DMSO-d6) δ 174.84, 168.40, 160.95, 155.78, 143.68, 137.93, 133.62, 133.36, 133.11, 129.87, 129.87, 128.32, 127.61, 125.24, 124.57, 123.81, 123.81, 122.73, 122.21, 120.00, 119.22; HRMS (ESI) calcd for C21H11N3O2F6Cl; [M+H]+: 486.04385, found 486.04257.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-isopropyl-1,2,4-oxadiazole (5p) White solid; yield 56.1%; mp 88.5–89.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.63 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.55–8.54 (m, 1H, Pyridine-H), 8.09 (d, J = 8.8 Hz, 2H, Ph-H), 7.47–7.43 (m, 2H, Ph-H), 2.81 (d, J = 63.7 Hz, 1H, –CH–), 1.39 (d, J = 7.0 Hz, 6H, –CH3). 13C NMR (101 MHz, DMSO-d6) δ 184.70, 167.30, 160.89, 155.43, 143.63, 137.83, 129.75, 129.19, 126.50, 124.78, 124.22, 123.01, 122.48, 122.11, 119.13, 27.28, 20.29; HRMS (ESI) calcd for C17H14N3O2F3Cl [M+H]+: 384.07212, found 384.07095.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-ethyl-1,2,4-oxadiazole (5q) White solid; yield 44.8%; mp 83.3–84.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.63 (d, J = 2.0 Hz, 1H, Pyridine-H), 8.55 (d, J = 1.1 Hz, 1H, Pyridine-H), 8.09 (d, J = 8.7 Hz, 2H, Ph-H), 7.45 (d, J = 8.7 Hz, 2H, Ph-H), 3.03 (d, J = 7.6 Hz, 2H, –CH2–), 1.35 (t, J = 7.6 Hz, 3H, –CH3–). 13C NMR (101 MHz, DMSO-d6) δ 181.81, 167.34, 160.91, 155.42, 143.62, 137.84, 129.77, 129.15, 124.79, 124.23, 123.02, 122.46, 122.10, 119.12, 20.07, 10.92; HRMS (ESI) calcd for C16H12N3O2F3Cl [M+H]+: 370.05647, found 370.05554.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-(chloromethyl)-1,2,4-oxadiazole (5r) White solid; yield 46.7%; mp: 94.6–95.7 °C; 1H NMR (500 MHz, DMSO-d6) δ 8.59 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.51 (dd, J = 1.9, 0.8 Hz, 1H, Pyridine-H), 8.10–8.05 (m, 2H, Ph-H), 7.46–7.42 (m, 2H, Ph-H), 5.17 (s, 2H, –CH2–). 13C NMR (126 MHz, DMSO-d6) δ 176.24, 167.98, 160.93, 155.82, 143.69, 138.04, 137.82, 129.39, 124.57, 123.58, 123.41, 122.05, 120.00, 119.23, 34.26; HRMS (ESI) calcd for C15H9N3O2F3Cl2 [M+H]+: 390.00184, found 390.00082.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-((2,4-dichlorophenoxy)methyl)-1,2,4-oxadiazole (5 s) Yellow solid; yield 49.0%; mp: 97.1–98.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.59 (d, J = 2.2 Hz, 1H, Pyridine-H), 8.50 (s, 1H, Pyridine-H), 8.06 (d, J = 8.7 Hz, 2H, Ph-H), 7.62 (d, J = 2.5 Hz, 1H, Ph-H), 7.45–7.42 (m, 2H, Ph-H), 7.40 (dd, J = 8.9, 2.6 Hz, 1H, Ph-H), 7.32 (d, J = 9.0 Hz, 1H, Ph-H), 5.72 (s, 2H, –CH2–). 13C NMR (101 MHz, DMSO-d6) δ 175.82, 167.71, 160.94, 155.77, 152.45, 152.27, 143.67, 137.92, 130.19, 130.19, 128.78, 126.52, 124.57, 123.64, 123.63, 123.63, 122.67, 122.20, 119.21, 116.34, 62.50; HRMS (ESI) calcd for C21H12N3O3F3Cl3 [M+H]+: 515.98779, found 515.98909.
4-((3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-1,2,4-oxadiazol-5-yl)methoxy)benzonitrile (5t) White solid; yield 61.8%; mp: 119.0–120.2 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.59 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.50 (d, J = 1.1 Hz, 1H, Pyridine-H), 8.06 (d, J = 8.7 Hz, 2H, Ph-H), 7.82 (d, J = 8.9 Hz, 2H, Ph-H), 7.44 (d, J = 8.7 Hz, 2H, Ph-H), 7.26 (d, J = 8.9 Hz, 2H, Ph-H), 5.72 (s, 2H, –CH2–). 13C NMR (101 MHz, DMSO-d6) δ 175.81, 167.72, 160.93, 155.78, 143.68, 137.95, 134.94, 134.94, 129.42, 129.42, 124.57, 123.62, 123.22, 123.22, 122.63, 122.21, 119.39, 119.22, 116.54, 116.36, 104.80, 61.61; HRMS (ESI) calcd for C22H13N4O3F3Cl [M+H]+: 473.06228, found 473.06107.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-((4-fluorophenoxy)methyl)-1,2,4-oxadiazole (5u) Yellow solid; yield 41.8%; mp: 101.1–102.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.63 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.55–8.53 (m, 1H, Pyridine-H), 8.13–8.09 (m, 2H, Ph-H), 7.49–7.45 (m, 2H, Ph-H), 7.21–7.11 (m, 4H, Ph-H), 5.60 (s, 2H, –CH2–). 13C NMR (101 MHz, DMSO-d6) δ 176.30, 167.61, 160.88, 158.86, 156.50, 155.69, 154.06, 143.61, 137.85, 129.34, 129.34, 124.78, 123.65, 123.14, 123.14, 122.52, 122.13, 119.16, 116.99, 116.99, 61.91; HRMS (ESI) calcd for C21H13N3O3F4Cl [M+H]+: 466.05761, found 466.05652.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-((4-chlorophenoxy)methyl)-1,2,4-oxadiazole (5v) Brown solid; yield 48.5%; mp: 92.3–93.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.64 (d, J = 2.0 Hz, 1H, Pyridine-H), 8.55 (dd, J = 2.1, 0.9 Hz, 1H, Pyridine-H), 8.12–8.09 (m, 2H, Ph-H), 7.49–7.46 (m, 2H, Ph-H), 7.42–7.39 (m, 2H, Ph-H), 7.16–7.13 (m, 2H, Ph-H), 5.64 (s, 2H, –CH2–). 13C NMR (101 MHz, DMSO-d6) δ 176.16, 167.62, 160.88, 156.59, 155.70, 143.63, 137.87, 129.95, 129.95, 129.35, 126.11, 124.79, 123.62, 123.17, 123.17, 122.50, 122.13, 119.15, 117.13, 117.13, 61.59; HRMS (ESI) calcd for C21H13N3O3F3Cl2 [M+H]+: 482.02806, found 482.02698.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-((p-tolyloxy)methyl)-1,2,4-oxadiazole (5w) Yellow solid; yield 54.9%; mp: 84.2–84.7 °C; 1H NMR (500 MHz, DMSO-d6) δ 8.59 (d, J = 2.1 Hz, 1H, Pyridine-H), 8.50 (dd, J = 1.9, 0.8 Hz, 1H, Pyridine-H), 8.09–8.05 (m, 2H, Ph-H), 7.45–7.41 (m, 2H, Ph-H), 7.10 (d, J = 8.2 Hz, 2H, Ph-H), 6.96–6.93 (m, 2H, Ph-H), 5.53 (s, 2H, –CH2–), 2.20 (s, 3H, –CH3). 13C NMR (126 MHz, DMSO-d6) δ 176.62, 167.65, 160.94, 155.72, 143.68, 137.94, 131.22, 130.55, 130.55, 129.40, 129.40, 124.57, 123.74, 123.21, 123.21, 122.62, 122.2, 119.22, 115.18, 115.18, 61.39, 20.61; HRMS (ESI) calcd for C22H16N3O3F3Cl; [M+H]+: 462.08268, found 462.08160.
4-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-((o-tolyloxy)methyl)-1,2,4-oxadiazole (5x) Yellow solid; yield 42.2%; mp: 80.0–80.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 8.60 (d, J = 2.0 Hz, 1H, Pyridine-H), 8.51 (s, 1H, Pyridine-H), 7.44 (d, J = 8.7 Hz, 3H, Ph-H), 7.16 (dd, J = 13.7, 7.4 Hz, 2H, Ph-H), 7.05 (d, J = 8.0 Hz, 1H, Ph-H), 6.89 (t, J = 7.3 Hz, 1H, Ph-H), 5.59 (s, 2H, –CH2–), 2.20 (s, 3H, -CH3). 13C NMR (126 MHz, DMSO-d6) δ 176.70, 167.67, 160.95, 155.98, 155.75, 143.69, 137.95, 131.37, 129.40, 129.40, 127.62, 126.80, 124.58, 123.75, 123.23, 123.23, 122.67, 121.90, 119.21, 112.57, 61.68, 16.45; HRMS (ESI) calcd for C22H16N3O3F3Cl [M+H]+: 462.08268, found 462.08160.
4-((3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-1,2,4-oxadiazol-5-yl)methoxy)-2-fluorobenzonitrile (5y) Red solid; yield 43.7%; mp: 114.7–115.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.59 (d, J = 2.2 Hz, 1H, Pyridine-H), 8.50 (d, J = 1.0 Hz, 1H, Pyridine-H), 8.08–8.04 (m, 2H, Ph-H), 7.92–7.87 (m, 1H, Ph-H), 7.45–7.42 (m, 2H, Ph-H), 7.38 (dd, J = 11.6, 2.4 Hz, 1H, Ph-H), 7.14 (dd, J = 8.8, 2.4 Hz, 1H, Ph-H), 5.75 (s, 2H, –CH2–). 13C NMR (101 MHz, DMSO-d6) δ 175.34, 167.65, 160.87, 155.72, 143.61, 137.87, 135.49, 129.36, 129.36, 124.77, 123.58, 123.35, 123.35, 122.07, 119.15, 114.63, 113.14, 104.17, 104.03, 103.59, 93.50, 62.12; HRMS (ESI) calcd for C22H12N4O3F4Cl [M+H]+: 491.05286, found 491.05167.
3-(4-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)-5-ethyl-1,2,4-oxadiazole (5z) White solid; yield 57.5%; mp: 105.2–106.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.64 (d, J = 2.0 Hz, 1H, Pyridine-H), 8.55 (d, J = 1.1 Hz, 1H, Pyridine-H), 8.54 (dd, J = 2.1, 0.9 Hz, 2H, Ph-H), 8.13–8.09 (m, 2H, Ph-H), 7.50–7.45 (m, 2H, Ph-H), 7.25–7.19 (m, 2H, Ph-H), 5.67 (s, 2H, –CH2–). 13C NMR (101 MHz, DMSO-d6) δ 176.12, 167.63, 160.88, 156.57, 155.70, 143.62, 143.11, 137.89, 137.87, 129.36, 129.36, 124.78, 123.62, 123.20, 123.20, 122.51, 122.13, 121.87, 119.24, 116.68, 116.68, 61.75; HRMS (ESI) calcd for C22H13N3O4F6Cl [M+H]+: 532.04933, found 532.04822.
Nematocidal activity
The nematocidal activity of the target compound was carried out according to the reported method [33]. The tomato grown in the greenhouse for cultivating southern root-knot nematodes were uprooted and washed with water. Then take the eggs of the roots with a toothpick and place them in a petri dish containing distilled water. Second instar larvae were collected after 3–7 days of incubation at 27 °C. All tested compounds were dissolved in DMF and diluted with 1% Tween 80 (final concentration of DMF was 0.5%). 250 μL of the test solution was added to a 48-well biochemical culture dish and tested. Subsequently, approximately 100 nematodes were added to each well. Abamectin was used as a positive control, and a test solution containing no compound was used as a negative control. After 48 h of treatment with the compound, the nematode was transferred to clear water for resuscitation, and the nematode that did not move was considered dead.
Antifungal assay
The mycelium growth rate method was utilized to evaluate in vitro antifungal activities of target compounds against R. solani [34]. The DMSO solution of the test compound was added to a sterilized petri dish containing about 10 mL of molten potato dextrose agar (PDA). Subsequently, a mycelial plug with a diameter of 4 mm was cut from the fungal colony and placed in the center of the PDA plate at 28 ± 1 °C for 4 days. For each compound, antifungal assays were performed in triplicate. In addition, pure DMSO and commercial fungicide (Hymexazol) were also used as negative and positive control agents, respectively.
The inhibition rate (I) of the tested compound was determined based on the following formula:
In the formula, C represents the average mycelial diameter of negative control and T represents the average mycelial diameter of tested compound-treated PDA.
Antibacterial activity in vitro
The previously described method was used for in vitro antibacterial activity testing [35,36,37]. The 50 μL culture of Xoo or Xoc in logarithmic growth phase were added to the test tubes with 5 mL of NB medium containing different concentrations of the target compound, respectively. The commercial bacteriacide thiodiazole copper (TDC, 20% suspending agent) and bismerthiazol (BMT, 20% wettable powder) as positive controls, while the same treatment without compound as the negative control. Then, the absorbance at 600 nm was measured when the tubes were inoculated at 28 °C for 48 h with shaking at 180 rpm. The inhibition rate (the inhibition rate refers to the proportion of bacteria whose growth was inhibited) was calculated by the following equation: where the absorbance of the negative control group was expressed as ODCK, and the absorbance of the treated group was expressed as ODT.
Antibacterial activity in vivo
In vivo biometric against rice bacterial leaf blight. The curative and protection activities of compound 5v against rice bacterial leaf blight were determined by Schaad’s method with some slight modifications [4]. The curative activity of the rice plant bacterial leaf blight-reducing compound 5v in potted plants was determined under controlled conditions in the growth room. About 8 weeks after planting the “Fengyouxiangzhan” rice seeds, Xoo was inoculated on the rice leaves. One day after the inoculation, 200 μg/mL 5v solutions was evenly sprayed onto the rice leaves until dripping, and 200 μg/mL BMT and TDC solutions, and distilled water was evenly sprayed as positive and negative control groups, respectively. Then, all inoculated rice plants were placed in a plant growth chamber (28 °C and 90% relative humidity). On the 14th day after spraying, the disease index of the inoculated rice leaves was measured. Similarly, the protective activity of reducing rice bacterial leaf blight of compound 5v was also evaluated, the difference is that 1 day after spraying the compound solution and distilled water, Xoo was inoculated on rice leaves and the disease index of the inoculated rice leaves was measured on the 14th day after inoculation. First, measure the spot area of each leaf and the entire leaf area, and then calculate the percentage of the spot area in the entire leaf area. Secondly, these leaves were classed according to the following grading standards. Grade 1: the area of disease spot accounts for less than 5% of the whole leaf area; Grade 3: the area of disease spot accounts for 6–10% of the whole leaf area; Grade 5: the area of disease spot accounts for 11–20% of the whole leaf area; Grade 7: the area of disease spot 6 accounts for 21–50% of the whole leaf area; Grade 9: the area of disease spot accounts for more than 50% of the whole leaf area. Finally, the disease index (C or T) was calculated using the following formula: Disease index (C or T) = ∑ (the number of leaves at each Grade × the corresponding Grade)/(the total number of leaves × the superlative Grade). The control coefficients I (%) for the curative and protection activities are calculated by the following equation. In the equation, C is the disease index of the negative control and T is the disease index of the treatment group.
Results and discussion
Design of novel 1,2,4-oxadiazole derivatives
1,2,4-Oxadiazole heterocycle is an important pharmacophore to design novel drug molecules. The compounds containing 1,2,4-oxadiazole skeleton possess various bioactivity in agricultural, including antibacterial, antifungal and nematocidal activities. Of which, tioxazafen is a new nematicide with unique mechanism of action developed by Monsanto. In our previous works, some 1,3,4-thiadiazol or 1,3,4-oxadiazole derivatives were designed and synthesized, and they exhibited good antibacterial, antifungal and nematocidal activities. So, we firstly introduced 1,3,4-thiadiazol or 1,3,4-oxadiazole into 1,2,4-oxadiazole skeleton to find highly active compounds. Meanwhile, the literature survey reveals that fluopyram showed good antibacterial activity and nematocidal activity, and the important pharmacophore is trifluoromethyl pyridine moiety. Encouraged by this results, we designed the novel 1,2,4-oxadiazole derivatives containing a trifluoromethyl pyridine moiety to find new lead compounds.
Chemistry
1H NMR, 13C NMR, and HRMS were used to characterize the physical properties of the target compounds 5a–5z. 1H NMR, 13C NMR, and HRMS data are provided in Additional file 1. In 1H NMR spectra of compound 5v, singlet at δ 8.64–8.55 ppm reveals the presence of Pyridine-H protons, singlet at δ 8.12–7.13 ppm reveals the presence of Ph-H protons. From the analysis of the 13C NMR spectrum of the compound 5v, it can be seen that 176.16 and 167.62 ppm are the absorption peaks of carbon on oxadiazole structure, 160.88, 156.59 and 155.70 ppm are the absorption peaks of carbon on the benzene ring directly connected to the oxygen group, and 61.59 ppm is the absorption peak of methylene carbon.
Nematocidal activity screening of title compounds
The in vitro nematocidal activity of the target compounds 5a–5i was evaluated using the direct strike method against Meloidogyne incognita. The results showed that all of the 1,2,4-oxadiazole derivatives containing 1,3,4-thiadiazol or 1,3,4-oxadiazole moiety have low nematocidal activities. And then, introducing the trifluoromethyl pyridine moiety can enhance the activity. Of which, compounds 5n and 5v exhibited significant nematocidal activity against M. incognita, with the inhibitory ratio of 63.3% and 55.0% at 100 μg/mL, respectively, which was superior to that of tioxazafen (29.0%). There was no good activity of the 1,2,4-oxadiazole derivatives containing trifluoromethyl pyridine and diether groups (Table 1).
Antifungal activity screening of title compounds
Antifungal activity of target compounds was evaluated by using the mycelium growth method. Unfortunately, the results revealed that almost all the compounds failed to exhibit a noticeable fungicidal activity (≥ 50.0%) against R. solani at 50 μg/mL (Table 1).
Antibacterial activity screening of title compounds
In vitro bacterial activity test was performed using the turbidity method and the results were listed in Table 2. As shown in Table 2, thioether derivatives containing an 1,2,4-oxadiazole scaffold have low antibacterial activities. Some target compounds introducing the trifluoromethyl pyridine moiety showed better antibacterial activities against Xoo and Xoc at a concentration of 50 μg/mL compared to the control drugs, for example compounds 5m, 5r, 5u, 5v, 5x, and 5y with the values of 65.85, 71.89, 64.97, 85.37, 61.97, and 78.44% against Xoo, respectively. Meanwhile, Compounds 5p, 5u, and 5v with the values of 64.53, 65.10, and 64.59% against Xoo, respectively. The half maximal effective concentration (EC50) value of compounds was further tested. The results clearly showed that some target compounds exhibit better antibacterial activity than that of bismerthiazol (BMT) and thiodiazole copper (TDC). Compounds 5m, 5r, 5u, 5v, 5x and 5y showed excellent antibacterial effects on Xoo, with EC50 values of 36.25, 24.14, 28.82, 19.44, 25.37 and 28.52 μg/mL, respectively, stronger than BMT (EC50 = 77.46 μg/mL) and TDC (EC50 = 99.31 μg/mL). Compounds 5n, 5p, 5t, 5u, 5v and 5z exhibited strong antibacterial ability against Xoc, with EC50 values of 50.93, 31.40, 56.50, 19.04, 21.78, and 55.32 μg/mL, respectively, superior to the control agents BMT (EC50 = 68.50 μg/mL) and TDC (EC50 = 91.05 μg/mL). Among these target compounds, compound 5v and 5u showed the best antibacterial activity on Xoo and Xoc, respectively.
Compound 5v exhibited excellent antibacterial ability to Xoo, with an EC50 value of 19.44 μg/mL. Accordingly, the control effect of compound 5v on rice bacterial leaf blight was evaluated and the results were showed in Table 3 and Fig. 2. Compound 5v exerted moderate control effects on rice bacterial leaf blight at 200 μg/mL, with curative and protective activity values of 37.8% and 27.6%, respectively, lower than those of the control agents BMT (46.7% and 36.1%, respectively) and TDC (31.8% and 30.9%, respectively).
The preliminary structure–activity relationship analysis results were as follows: Firstly, the introduction of strong electron withdrawing groups, such as –NO2 (5n, EC50 = 50.93 μg/mL) and 4-CF3 (5o, EC50 = 108.27 μg/mL), into the benzene ring group of mono-ether structure compounds 5j–5r, culminated in either a significant or non-significant improvement in antibacterial activity; alkyl groups did not evoke a significant improvement over phenyl. Secondly, compared to other substituents, we found that the introduction of fluorine or chlorine (compounds 5u and 5v) at the 4-position of the phenyl group exerted the most significant effect on improving antibacterial activity against Xoo and Xoc; confusingly, continue compounds 5v to introduce chlorine atom (such as compound 5 s with 2,4-Cl, EC50 = 116.76 μg/mL) on the benzene ring second position was overall antibacterial ability has not improved but has declined. Finally, introduction of electron withdrawing group or electron donating group of thioether structure compounds 5a–5i disfavor to their activities.
Conclusions
In summary, twenty-six novel 1,2,4-oxadiazole derivatives containing 1,3,4-thiadiazol (1,3,4-oxadiazole) and trifluoromethyl pyridine moieties were synthesized based on 1,2,4-oxadiazole pharmacophore. Compound 5n and 5v exhibited excellent nematocidal activity, superior to leading compound Tioxazafen. What’s more notable is that compound 5v and 5u showed significantly inhibitory activity against the plant pathogenic bacteria Xoo and Xoc, respectively, higher than commercial bactericide BMT and TDC. The present study demonstrated the potential of 1,2,4-oxadiazole ether derivatives as effective nematocidal and antimicrobial agents for crop protection and should serve as a basis for future studies.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Additional files].
Abbreviations
- Xoo :
-
Xanthomonas oryzae pv. oryzae
- Xoc :
-
Xanthomonas oryzae pv. oryzicola
- BMT:
-
Bismerthiazol
- TDC:
-
Thiodiazole copper
- 1H NMR:
-
1H nuclear magnetic resonance
- 13C NMR:
-
13C nuclear magnetic resonance
- HRMS:
-
High resolution mass spectrum
References
Zou LF, Li YR, Chen GY (2011) A non-marker mutagenesis strategy to generate poly-hrp gene mutants in the rice pathogen Xanthomonas oryzae pv. oryzicola. Agric Sci China 10:1139–1150
Graham JH, Gottwald TR, Cubero J, Achor DS (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 5:1–15
Yi CF, Chen JX, Wei CQ, Wu SK, Wang SB, Hu DY, Song BA (2020) α-Haloacetophenone and analogues as potential antibacterial agents and nematicides. Bioorg Med Chem Lett 30:126814–126818
Li P, Hu DY, Xie DD, Chen JX, Jin LH, Song BA (2018) Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J Agric Food Chem 66:3093–3100
Shi J, Luo N, Ding MH, Bao XP (2019) Synthesis, in vitro antibacterial and antifungal evaluation of novel 1,3,4-oxadiazole thioether derivatives bearing the 6-fluoroquinazolinylpiperidinyl moiety. Chin Chem Lett 31:434–438
Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchi EGJ, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Segurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, Schiex T, Smant G, Weissenbach J, Wincker P (2008) Genomese quence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909–915
Abad P, Favery B, Rosso MN, Castagnone SP (2003) Root-knot nematode parasitism and host response: molecular basis of a sophisticated interaction. Mol Plant Pathol 4:217–224
Djian-Caporalino C, Fazari A, Arguel MJ, Vernie T, VandeCasteele C, Faure I, Brunoud G, Pijarowski L, Palloix P, Lefebvre V, Abad P (2007) Root-knot nematode (Meloidogyne spp.) Me resistance genes in pepper (Capsicum annuum L.) are clustered on the P9 chromosome. Theor Appl Genet 114:473–486
Seenivasan N (2017) Status of root-knot nematode, Meloidogyne hapla infection on carrot at Kodaikanal hills of Tamil Nadu, India and its yield loss estimation. Int J Curr Microbiol App Sci 6:1–7
Meher HC, Gajbhiye VT, Chawla G, Singh GD (2009) Virulence development and genetic polymorphism in Meloidogyne incognita (Kofoid & White) Chitwood after prolonged exposure to sublethal concentrations of nematicides and continuous growing of resistant tomato cultivars. Pest Manag Sci 65:1201–1207
Caboni P, Aissani N, Cabras T, Falqui AB, Marotta RB, Liori B, Ntalli NK, Sarais G, Sasanelli N, Tocco G (2013) Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita. J Agric Food Chem 61:1794–1803
Jang JY, Dang QL, Choi YH, Choi GJ, Jang KS, Cha B, Luu NH, Kim JC (2015) Nematicidal activities of 4-quinolone alkaloids isolated from the aerial part of Triumfetta grandidens against Meloidogyne incognita. J Agric Food Chem 63:68–74
Shafikova TN, Omelichkina YV (2015) Molecular-genetic aspects of plant immunity to phytopathogenic bacteria and fungi. Russ J Plant Physiol 62:571–585
Qiao M, Ying GG, Singer AC, Zhu YG (2018) Review of antibiotic resistance in China and its environment. Environ Int 110:160–172
Pan XY, Xu S, Wu J, Luo JY, Duan YB, Wang JX, Zhang F, Zhou MG (2018) Screening and characterization, of Xanthomonasoryzae pv. oryzae strains with resistance to pheazine-1-carboxylic acid. Pestic Biochem Phys 145:8–14
Wang PY, Zhou L, Zhou J, Wu ZB, Xue W, Song BA, Yang S (2016) Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4-oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg Med Chem Lett 26:1214–1217
Xu WM, Li SZ, He M, Yang S, Li XY, Li P (2013) Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg Med Chem Lett 23:5821–5824
Aslam MN, Mukhtar T, Ashfaq M, Hussain MA (2017) Evaluation of chili germplasm for resistance to bacterial wilt caused by Ralstonia solanacearum. Australas Plant Path 46:289–292
Devendar P, Yang GF (2017) Sulfur-containing agrochemicals. Top Curr Chem (Z) 375:82
Dahl BH, Peters D, Olsen GM, Timmermann DB, Joergensen S (2006) Novel oxadiazole derivatives as nicotinic acetylcholine receptor modulators and their preparation, pharmaceutical compositions, and use in the treatment of various central nervous system and peripheral nervous system diseases WO 2006114400 A1
Dai H, Chen J, Li G, Ge S, Shi Y, Fang Y, Ling Y (2017) Design, synthesis, and bioactivities of novel oxadiazole-substituted pyrazole oximes. Bioorg Med Chem Lett 27:950–953
Liu Q, Zhu R, Gao S, Ma SH, Tang HJ, Yang JJ, Diao YM, Wang HL, Zhu HJ (2017) Structure-based bioisosterism design, synthesis, insecticidal activity and structure-activity relationship (SAR) of anthranilic diamide analogues containing 1,2,4-oxadiazole rings. Pest Manag Sci 73:917–924
Sheng R, Li S, Lin GY, Shangguan SH, Gu YC, Qiu N, Cao J, He QJ, Yang B, Hu YZ (2015) Novel potent HIF-1 inhibitors for the prevention of tumor metastasis: discovery and optimization of 3-aryl-5-indazole-1,2,4-oxadiazole derivatives. Rsc Adv 5:81817–81830
Takahashi H, Riether D, Bartolozzi A, Bosanac T, Berger V, Binetti R, Broadwater J, Chen ZD, Crux R, Lombaert SD, Dave R, Dines JA, Fadra-Khan T, Flegg A, Garrigou M, Hao MH, Huber J, Hutzler JM, Kerr S, Kotey A, Liu WM, Lo HY, Loke PL, Mahaney PE, Morwick TM, Napier S, Olague A, Pack E, Padyana AK, Thomson DS, Tye H, Wu LF, Zindell RM, Abeywardane A, Simpson T (2015) Synthesis, SAR, and series evolution of novel oxadiazole-containing 5-lipoxygenase activating protein inhibitors: discovery of 2-[4-(3-{(R)-1-[4-(2-amino-pyrimidin-5-yl)-phenyl]-1-cyclopropyl-ethyl}-[1,2,4]oxadiazol-5-yl)-pyrazol-1-yl]-N, N-dimethyl-acetamide(BI665915). J Med Chem 58:1669–1690
Li P, Shi L, Yang X, Yang L, Chen XW, Wu F, Shi QC, Xu WM, He M, Hu DY, Song BA (2014) Design, synthesis, and antibacterial activity against rice bacterial leaf blight and leaf streak of 2,5-substituted-1,3,4-oxadiazole/thiadiazole sulfone derivative. Bioorg Med Chem Lett 24:1677–1680
Xu WM, Han FF, He M, Hu DY, He J, Yang S, Song BA (2012) Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiaole. J Agric Food Chem 60:1036–1041
Xu WM, He J, He M, Han FF, Chen XH, Pan ZX, Wang J, Tong MG (2011) Synthesis and antifungal activity of novel sulfone derivatives containing 1,3,4-oxadiazole moieties. Molecules 16:9129–9141
Chen Q, Zhu XL, Jiang LL, Liu ZM, Yang GF (2008) Synthesis, antifungal activity and CoMFA analysis of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives. Eur J Med Chem 43:595–603
Zhang MZ, Mulholland N, Beattie D, Irwin D, Gu YC, Chen Q, Yang GF, Clough J (2013) Synthesis and antifungal activity of 3-(1,3,4-oxadiazol-5-yl)-indoles and 3-(1,3,4-oxadiazol-5-yl)methyl-indoles. Eur J Med Chem 63:22–32
Chen JX, Chen YZ, Gan XH, Song BJ, Hu DY, Song BA (2018) Synthesis, nematicidal evaluation, and 3D-QSAR analysis of novel 1,3,4-oxadiazole–cinnamic acid hybrids. J Agric Food Chem 66:9616–9623
Abad-Feuntes A, Ceballos-Alcantrallia E, Mercader JV, Agullό C, Abad-Somovilla A, Esteve-Turrillas FA (2015) Determination of succinate dehydrogenase-inhibitor fungicide residues in fruits and vegetables by liquid-chromatography-tandem mass spectrometry. Anal Bioanal Chem 407:4207–4211
Jones JG, Kleczewski NM, Desaeger J, Meyer SL, Johnson GC (2017) Evaluation of nematicides for southern root-knot nematode management in lima bean. Crop Protect 96:151–157
Chen JX, Yi CF, Wang SB, Wu SK, Li SY, Hu DY, Song BA (2019) Novel amide derivatives containing 1,3,4-thiadiazole moiety: design, synthesis, nematocidal and antibacterial activities. Bioorg Med Chem Lett 29:1203–1210
Fan ZJ, Shi J, Luo N, Ding MH, Bao XP (2019) Synthesis, crystal structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1,2,4-triazolo[4,3-a]pyridine moiety. J Agric Food Chem 67:11598–11606
Jung J, Zhao YY (2013) Impact of the structural differences between α- and β-chitosan on their depolymerizing reaction and antibacterial activity. J Agric Food Chem 61:8783–8789
Zhu XF, Xu Y, Peng D, Zhang Y, Huang TT, Wang JX, Zhou MG (2013) Detection and characterization of bismerthiazol-resistance of Xanthomonas oryzae pv. oryzae. Crop Prot 47:24–29
Wang MW, Zhu HH, Wang PY, Zeng D, Wu YY, Liu LW, Wu ZB, Li Z, Yang S (2019) Synthesis of thiazolium-labeled 1,3,4-oxadiazole thioethers as prospective antimicrobials: in vitro and in vivo bioactivity and mechanism of action. J Agric Food Chem 67:12696–12708
Acknowledgements
Not applicable.
Funding
The authors gratefully acknowledge assistance from the National Key Research Development Program of China (2017YFD0201404 and 2018YFD0200100), the National Nature Science Foundation of China (32060622), the Outstanding Young Scientific and Technological Talents Project of Guizhou Province ([2019]5646), and the Construction Project of Key Laboratories from the Education Department of Guizhou Province (QJHKY [2018] 001).
Author information
Authors and Affiliations
Contributions
The current study is an outcome of constructive discussion with BS and XG. LZ, XG and QW carry out their synthesis and characterization experiments; LZ, HZ, DL, YF, and QW performed the biological activities; LZ, XG, HZ, DL, YF, and QW carried out the 1H NMR, 13C NMR and HRMS spectral analyses; LZ and XG were involved in the drafting of the manuscript and revising the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental research was performed following the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora and it was approved by Subcommittee of Experimental Animal Ethics, and Center for Research and Development of Fine Chemicals of Guizhou University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
The 1H NMR, 13C NMR, and HRMS data of target compounds.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, L., Zeng, H., Liu, D. et al. Design, synthesis, and biological activity of novel 1,2,4-oxadiazole derivatives. BMC Chemistry 14, 68 (2020). https://doi.org/10.1186/s13065-020-00722-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-020-00722-1