Abstract
Background
Take-all of wheat, caused by the soil-borne fungus Gaeumannomyces graminis var. tritici, is one of the most important and widespread root diseases. Given that take-all is still hard to control, it is necessary to develop new effective agrochemicals. Pyrazole derivatives have been often reported for their favorable bioactivities. In order to discover compounds with high fungicidal activity and simple structures, 1,2,3,4-tetrahydroquinoline, a biologically active group of natural products, was introduced to pyrazole structure. A series of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline were synthesized, and their fungicidal activities were evaluated.
Results
The bioassay results demonstrated that the title compounds displayed obvious fungicidal activities at a concentration of 50 μg/mL, especially against V. mali, S. sclerotiorum and G. graminis var. tritici. The inhibition rates of compounds 10d, 10e, 10h, 10i and 10j against G. graminis var. tritici were all above 90 %. Even at a lower concentration of 16.7 μg/mL, compounds 10d and 10e exhibited satisfied activities of 100 % and 94.0 %, respectively. It is comparable to that of the positive control pyraclostrobin with 100 % inhibition rate.
Conclusion
A series of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline were synthesized and their structures were confirmed by 1H NMR, 13C NMR, IR spectrum and HRMS or elemental analysis. The crystal structure of compound 10g was confirmed by X-ray diffraction. Bioassay results indicated that all title compounds exhibited obvious fungicidal activities. In particular, compounds 10d and 10e showed comparable activities against G. graminis var. tritici with the commercial fungicide pyraclostrobin at the concentration of 16.7 μg/mL.

A series of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline were designed and synthesized. Bioassay results indicated that all these compounds exhibited obvious fungicidal activities.
Similar content being viewed by others
Background
Wheat (Triticum aestivum) is one of the most important crops in the world. Take-all of wheat, caused by the soil-borne fungus Gaeumannomyces graminis var. tritici, is one of the most serious and widespread root diseases [1, 2]. The pathogen infects the roots of susceptible plants, resulting in black necrotic, plant stunting, white heads, and etc. [3, 4]. It reduces the grain yield from 20 % up to 50 %. Unfortunately, the control of take-all is still a huge problem. And the application of agrochemicals is currently the most effective method [5]. However, existing chemical control agents, such as silthiopham, were not financially affordable for the control of wheat take-all [6]. Hence, it is necessary to develop effective and inexpensive agents to replace the conventional agrochemicals.
Introducing active groups of natural products is an effective and important method for the discovery of new agrochemicals [7, 8]. 1,2,3,4-tetrahydroquinoline (THQ), widely existing in natural products [9, 10], has been often reported for its favorable bioactivities, such as anticancer [11, 12], antibacterial [13, 14], antifungal [15, 16] activities, and so on. For example, aspernigerin (Fig. 1), isolated from the extract of a culture of Aspergillus niger IFB-E003, exhibited favorable cytotoxic to the tumor cell lines [17], and certain fungicidal activities, insecticidal activities and herbicidal activities [18, 19].
In recent years, pyrazole derivatives have attracted tremendous attention owing to their excellent bioactivities [20–22]. Pyraclostrobin (Fig. 1) discovered by BASF is a commercial fungicide containing pyrazole structure. It came to the market in 2002. Given its wide fungicidal spectrum, pyraclostrobin had achieved a total sale of $800 million in 2012, ranked the second in the world. [23]. Besides, pyrazole derivatives were also reported to possess insecticidal activities [24, 25], herbicidal activities [26], and anticancer activities [27, 28].
It is an effective method to develop new green agrochemicals by introducing active groups of natural products to known active sub-structures. As above mentioned, THQ is an important active group of natural products. In order to find highly biologically active lead compounds with simple structures, THQ was introduced to the known active sub-substructure of pyrazole compounds using intermediate derivatization methods (IDM) [29]. A series of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline were synthesized, and their activities were evaluated in this study. Biological assays revealed that some compounds exhibited good fungicidal activities. Especially, they displayed excellent activities against G. graminis var. tritici.
Results and discussion
Synthesis
The synthetic procedure of intermediates 3a–3n is shown in Scheme 1 [30]. By using Claisen condensation in the presence of sodium ethoxide, substituted ketone 1 reacted with diethyl oxalate to afford the β-ketoester intermediate 2. With glacial acetic acid acidification, compound 2 was reacted with substituted hydrazine via Knorr reaction to obtain the intermediates 3a–3n. This method has two advantages. Firstly, ethyl 5-pyrazolecarboxylate compounds were synthesized simply through a “one-pot” process. Secondly, the reaction proceeds well at ambient temperature.
Synthesis of compounds 3o–3p is carried out following a different method [31, 32] and the procedure was shown in Scheme 2. 2,3-dichloropyridine 4 reacted with hydrazine hydrate (80 %) to yield the intermediate 5, which underwent cyclization with diethyl maleate to give the intermediate 6. The reaction of 6 with phosphorus oxychloride or phosphorus oxybromide afforded the chlorine or bromine substituted compound 7, which was then oxidized to give the intermediates 3o–3p.
General synthetic procedure of title compounds 10a–10p is shown in Scheme 3. The saponification of the ester intermediate 3 afforded the substituted-1H-pyrazole-5-carboxylic acid 8 [33]. The title compounds 10 were prepared by the amidation of compounds 9 and 1,2,3,4-tetrahydroquinoline (THQ) [34].
The structures of all the title compounds were confirmed by 1H NMR, 13C NMR, IR spectra and HRMS or elemental analysis and the relevant data could be found in the Additional file 1. Compound 10a was taken as an example to analyze the 1H NMR spectra data. Four protons of the benzene ring were observed at δ 7.18–6.87. A single peak at δ 5.76 was due to the proton at the 4-position of the parazole ring. Two protons at the 2-position of THQ were observed at δ 3.90 with J = 6.5 Hz as a triple peak, and the other triple peak at δ 2.82 with J = 6.6 Hz was due to the protons at the 4-position of THQ. Two protons at the 3-position of THQ was showed at δ 2.03 with J = 6.6 Hz as pentaploid peaks. The chemical shifts as single peaks were observed at δ 3.87 and 2.15 due to the protons of N-CH3 and CH3 at the 3-position of the parazole ring respectively.
In order to further confirm the structure of the title compounds, a single crystal of 10g (R1 = Ph, R2 = Me) was prepared for the X-ray diffraction. The single crystal was obtained by slow evaporation of a solution of compound 10g in ethyl acetate at room temperature. As shown in Fig. 2, the crystal data for 10g: orthorhombic, space group P212121 (no. 19), a = 8.3512(9) Å, b = 12.5600(13) Å, c = 15.3638(16) Å, V = 1611.5(3) Å3, Z = 4, T = 180.01(10) K, μ(Mo Kα) = 0.083 mm−1, Dcalc = 1.308 g/mm3, 5965 reflections measured (5.858 ≤ 2Θ ≤ 52.042), 3141 unique (R int = 0.0292) which were used in all calculations. The final R 1 was 0.0369 (I > 2σ(I)) and wR 2 was 0.0852. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 1441750. For more information on crystal data, see the Additional files 2 and 3.
Biological activity
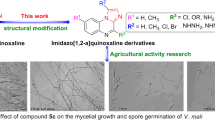
The in vitro fungicidal activities of all the title compounds have been determined against seven pathogenic fungi at the concentration of 50 μg/mL, and the mycelium growth rate method was used [35, 36]. Pyraclostrobin (Fig. 1) was assessed as a positive control. The bioassay results, illustrated in Table 1, indicated that the title compounds exhibited obvious fungicidal activities. Most of them displayed satisfied activities against V. mali, S. sclerotiorum and G. graminis var. tritici. Particularly, compounds 10d, 10e, 10i and 10j showed inhibitory activities of more than 85 % against V. mali. Compounds 10d, 10e, 10f, 10h, 10i, 10j and 10l also demonstrated good activities against S. sclerotiorum. Especially, five title compounds (10d, 10e, 10h, 10i and 10j) exhibited striking activities against G. graminis var. tritici, with more than 90 % inhibition rates.
Primary structure activity relationships (SAR) revealed that the substituents played an important role in fungicidal activities. (1) When substituent R1 was methyl, compounds with R2 as (substituted) phenyl exhibited better activities than those with R2 as alkyl (10d, 10e, 10f > 10a, 10b, 10c). (2) When R1 was phenyl, the fungicidal activities increased with the increase of the carbon number in the alkyl chain of the R2 moiety (10g < 10h < 10i ≈ 10j). However, fungicidal activities decreased dramatically when R1 and R2 were both phenyl (10k). (3) It was not beneficial to increase their fungicidal activities when R1 was substituted pyridyl (10o and 10p).
In particular, compounds 10d (R1 = Me, R2 = Ph), 10e (R1 = Me, R2 = 4-OMePh), 10i (R1 = Ph, R2 = n-Pr) and 10j (R1 = Ph, R2 = i-Pr) exhibited good activities against V. mali, S. sclerotiorum and G. graminis var. tritici with inhibition rates of more than 80 %. Compounds 10d and 10e showed comparable activities against V. mali and G. graminis var. tritici with the commercial fungicide pyraclostrobin.
In the further study, fungicidal activities against G. graminis var. tritici of compounds 10d, 10e, 10h, 10i and 10j were evaluated at lower concentrations (Table 2). Obviously, the result revealed a dosage-dependent relationship. Compounds 10d and 10e still exhibited satisfied activities with the inhibition rates of 100 % and 94.0 % at the concentration of 16.7 μg/mL, respectively, which is comparable to that of the positive control using pyraclostrobin. Unfortunately, their fungicidal activities decreased dramatically at the concentration of 11.1 μg/mL.
Experimental
Chemistry
Melting points of all compounds were determined on an X-4 binocular microscope (Fukai Instrument Co., Beijing, China) without calibration. NMR spectra were acquired with a Bruker 300 MHz spectrometer with CDCl3 as the solvent and TMS as the internal standard. Chemical shifts are reported in δ (parts per million) values. High resolution mass spectrometry (HRMS) data were obtained on an FTICR-MS Varian 7.0T FTICR-MS instrument. Elemental analysis was carried out on a Vario EL III elemental analyzer. All the reagents were obtained commercially and used without further purification. Column chromatography purification was carried out by using silica gel. The synthesis of intermediates and title compounds can be found in the Additional file 1.
Antifungal biological assay
All the target compounds have been evaluated for their in vitro fungicidal activities against seven pathogenic fungi, using mycelium growth rate method according to the literature [35, 36]. Fungi tested in this article included Pythium aphanidermatum, Rhizoctonia solani, Valsa mali, Sclerotinia sclerotiorum, Botrytis cinerea, Fusarium moniliforme and Gaeumannomyces graminis var. tritici. Dimethyl sulfoxide (DMSO) in sterile distilled water served as the control. Pyraclostrobin (Fig. 1) containing pyrazole structure (Fig. 1) as the commercial fungicide, was assessed under the same conditions as a positive control. In the preparation, every compound (10 mg) was weighted accurately and dissolved in 1 mL DMSO, and then it was mixed with 200 mL potato dextrose agar (PDA). As a consequence, they were tested at a concentration of 50 μg/mL. In order to get new mycelium for antifungal assay, all fungal species were incubated in PDA at 25 ± 1 °C for 1–7 days vary from different fungi. Mycelia dishes were cut with a 5 mm in diameter hole punch from the prepared edge of culture medium. One of them was picked up with a sterilized inoculation needle, and then inoculated in the center of the PDA plate aseptically. Every treatment repeated three times, and they were incubated at 25 ± 1 °C for 1–7 days vary from different fungi. All the above was completed in a bioclean environment. The hypha diameter was measured by a ruler, and the data were statistically analyzed. The inhibition rate of the title compounds on the fungi was calculated by the following formula:
I (%) = [(C − T)/(C − 5)] × 100, where I is the inhibition rate, C represents the diameter (mm) of fungal growth on untreated PDA, and T represents the diameter (mm) of fungi on treated PDA.
Conclusion
In summary, a series of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline were synthesized and their structures were confirmed by 1H NMR, 13C NMR, IR and HRMS or elemental analysis. The crystal structure of compound 10g was determined by X-ray diffraction. Bioassay results indicated that all the title compounds exhibited good fungicidal activities. And the substituents played an important role in fungicidal activities. In particular, compounds 10d and 10e with simple structures showed comparable activities against G. graminis var. tritici to the commercial fungicide pyraclostrobin even at the concentration 16.7 μg/mL. These two compounds could be valuable leads for further studies.
References
Keenan S, Cromey MG, Harrow SA, Bithell SL, Butler RC, Beard SS, Pitman AR (2015) Quantitative PCR to detect Gaeumannomyces graminis var. tritic in symptomatic and non-symptomatic wheat roots. Australas Plant Pathol 44:591–597
Chng S, Cromey MG, Dodd SL, Stewart A, Butler RC, Jaspers MV (2015) Take-all decline in New Zealand wheat soils and the microorganisms associated with the potential mechanisms of disease suppression. Plant Soil 397:239–259
Wang AY, Wei XN, Rong W, Dang L, Du LP, Qi L, Xu HJ, Shao YJ, Zhang ZY (2015) GmPGIP3 enhanced resistance to both take-all and common root rot diseases in transgenic wheat. Funct Integr Genomics 15:375–381
Toor RFV, Chng SF, Warren RM, Butler RC, Cromey MG (2015) The influence of growth stage of different cereal species on host susceptibility to Gaeumannomyces graminis var. tritici and on Pseudomonas populations in the rhizosphere. Australas Plant Pathol 44:57–70
Sun HY, Li Q, Du WZ, Guo YP, Zhang AX, Chen HG (2012) Control of wheat take-all by seed treatments with different fungicides. Plant Prot 38:155–158
Puga-Freitas R, Belkacem L, Barot S, Bertrand M, Roger-Estrade J, Blouin M (2016) Transcriptional profiling of wheat in response to take-all disease and mechanisms involved in earthworm’s biocontrol effect. Eur J Plant Pathol 144:155–165
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Lu AD, Wang JJ, Liu TJ, Han J, Li YH, Su M, Chen JX, Zhang H, Wang LZ, Wang QM (2014) Small changes result in large differences: discovery of (-)-incrustoporin derivatives as novel antiviral and antifungal agents. J Agric Food Chem 62:8799–8807
Sridharan V, Suryavanshi PA, Menéndez JC (2011) Advances in the chemistry of tetrahydroquinolines. Chem Rev 111:7157–7259
Keck D, Vanderheiden S, Bräse S (2006) A formal total synthesis of virantmycin: a modular approach towards tetrahydroquinoline natural products. Eur J Org Chem 21:4916–4923
Liou JP, Wu ZY, Kuo CC, Chang CY, Lu PY, Chen CM, Hsieh HP, Chang JY (2008) Discovery of 4-amino and 4-hydroxy-1-aroylindoles as potent tubulin polymerization inhibitors. J Med Chem 51:4351–4355
Chamberlain SD, Redman AM, Wilson JW, Deanda F, Shotwell JB, Gerding R, Lei HS, Yang B, Stevens KL, Hassell AM, Shewchuk LM, Leesnitzer MA, Smith JL, Sabbatini P, Atkins C, Groy A, Rowand JL, Kumar R, Mook RA Jr, Moorthy G, Patnaik S (2009) Optimization of 4,6-bis-anilno-1H-pyrrolo[2,3-d]pyrimidine IGF-1R tyrosine kinase inhibitors towards JNK selectivity. Bioorg Med Chem Lett 19:360–364
Jarvest RL, Berge JM, Berry V, Boyd HF, Brown MJ, Elder JS, Forrest AK, Fosberry AP, Gentry DR, Hibbs MJ, Jaworski DD, O’Hanlon PJ, Pope AJ, Rittenhouse S, Sheppard RJ, Slater-Radosti C, Worby A (2002) Nanomolar inhibitors of Staphylococcus aureus methionyl tRNA synthetase with potent antibacterial activity against gram-positive pathogens. J Med Chem 45:1959–1962
Jarvest RL, Armstrong SA, Berge JM, Brown P, Elder JS, Brown MJ, Copley RCB, Forrest AK, Hamprecht DW, O’Hanlon PJ, Mitchell DJ, Rittenhouse S, Witty DR (2004) Definition of the heterocyclic pharmacophore of bacterial methionyl tRNA synthetase inhibitors: potent antibacterially active non-quinolone analogues. Bioorg Med Lett 14:3937–3941
Urbina JM, Cortés JCG, Palma A, López SN, Zacchino SA, Enriz RD, Ribas JC, Kouznetzov VV (2000) Inhibitors of the fungal cell wall. Synthesis of 4-aryl-4-N-arylamine-1-butenes and related compounds with inhibitory activities on beta(1-3) glucan and chitin synthases. Bioorg Med Chem 8:691–698
Suvire FD, Sortino M, Kouznetsov VV, Vargas MLY, Zacchino SA, Cruz UM, Enriz RD (2006) Structure-activity relationship study of homoallylamines and related derivatives acting as antifungal agents. Bioorg Med Chem 14:1851–1862
Shen L, Ye YH, Wang XT, Zhu HL, Xu C, Song YC, Li H, Tan RX (2006) Structure and total synthesis of aspernigerin: a novel cytotoxic endophyte metabolite. Chemistry 12:4393–4396
Wu QL, Li YQ, Yang XL, Ling Y (2012) Facile total synthesis and bioactivity of aspernigerin. Chin J Org Chem 32:1498–1502
Wu QL, Li YQ, Yang XL, Ling Y (2012) Synthesis and bioactivity of aspernigerin analogues. Chin J Org Chem 32:747–754
Raghavendra KR, Girish YR, Ajay Kumar K, Shashikanth S (2015) Regioselective synthesis of N-formohydrazide and formyl pyrazole analogs as potent antimicrobial agents. J Chem Pharm Res 7:361–366
Xu H, Hu XH, Zou XM, Zhu YQ, Liu B, Hu FZ, Yang HZ (2012) Synthesis and herbicidal activity of 5-heterocycloxy-3-substituted-1-(3-trifluoromethyl) phenyl-1H-pyrazole. Chem Res Chin Univ 28:824–827
Zhang DQ, Xu GF, Liu YH, Wang DQ, Yang XL, Yuan DK (2015) Synthesis and biological study of novel N-(1H-pyrazol-4-yl)-1H-pyrazole-3(5)-carboxamides. Chin J Org Chem 35:2191–2198
Hu XX (2014) Study on the trends of global pesticide development and potentiality of key patent pesticides. Pestic Sci Adm 35:12–22
Lv XH, Xiao JJ, Ren ZL, Chu MJ, Wang P, Meng XF, Li DD, Cao HQ (2015) Design, synthesis and insecticidal activities of N-(4-cyano-1-phenyl-1H-pyrazol-5-yl)-1,3-diphenyl-1H-pyrazole-4-carboxamide derivatives. RSC Adv 5:55179–55185
Shi YJ, Wang SL, He HB, Li Y, Li Y, Fang Y, Dai H (2015) Synthesis and bioactivity of novel pyrazole oxime ester derivatives containing furan moiety. Chin J Org Chem 35:1785–1791
Ma HJ, Zhang JH, Xia XD, Xu MH, Ning J, Li JH (2014) Design, synthesis and herbicidal activities of novel 4-(1H-pyrazol-1-yl)-6-(alkynyloxy)-pyrimidine derivatives as potential pigment biosynthesis inhibitors. Pest Manag Sci 70:946–952
Vaarla K, Kesharwani RK, Santosh K, Vedula RR, Kotamraju S, Toopurani MK (2015) Synthesis, biological activity evaluation and molecular docking studies of novel coumarin substituted thiazolyl-3-aryl-pyrazole-4-carbaldehydes. Bioorg Med Chem Lett 25:5797–5803
Radi S, Tighadouini S, Feron O, Riant O, Bouakka M, Benabbes R, Mabkhot YN (2015) Synthesis of novel β-keto-enol derivatives tethered pyrazole, pyridine and furan as new potential antifungal and anti-breast cancer agents. Molecules 20:20186–20194
Guan AY, Liu CL, Yang XP, Dekeyser M (2014) Application of the intermediate derivatization approach in agrochemical discovery. Chem Rev 114:7079–7107
Liu M, Liu DK, Liu Y, Xu WR, Zhang SJ, Zhang CY, Tang LD (2009) Preparation of pyrazolo[1,5-a]pyridine derivatives as antitumor and/or antiviral agents. CN 101544634A
Xu JY, Dong WL, Xiong LX, Li YX, Li ZM (2009) Design, synthesis and biological activities of novel amides (sulfonamides) containing N-pyridylpyrazole. Chin J Chem 27:2007–2012
Zhao Y, Li YQ, Xiong LX, Wang HX, Li ZM (2012) Design, synthesis and biological activities of novel anthranilic diamide insecticide containing trifluoroethyl ether. Chin J Chem 30:1748–1758
Zhu HW, Wang BL, Zhang XL, Xiong LX, Yu SJ, Li ZM (2014) Syntheses and biological activities of novel 3-bromo-1-(3-chloropyridin-2-yl)-N-hydroxy-N-aryl-1H-pyrazole-5-carboxamides. Chem Res Chin Univ 30:409–414
Liu SH, Ling Y, Yang XL (2013) Synthesis, bioactivities and crystal structure of (Z)-N-(3-((2-(4-chlorophenyl)-oxazol-4-yl)methyl)thiazolidin-2-ylidene)cyanamide. Chin J Struct Chem 32:931–935
Xu Y, Lei P, Ling Y, Wang SW, Yang XL (2014) Design, synthesis and biological activity of novel (3-substituted phenyl-2-propen-1-ylidene)-benzoylhydrazones. Chin J Org Chem 34:1118–1123
Wu J, Kang SH, Luo LJ, Shi QC, Ma J, Yin J, Song BA, Hu DY, Yang S (2013) Synthesis and antifungal activities of novel nicotinamide derivatives containing 1,3,4-oxadiazole. Chem Cent J 7:64–69
Authors’ contributions
The current study is an outcome of constructive discussion with XLY and YL; PL carried out the synthesis, characterization and antifungal bioassay experiments and involved in the drafting of the manuscript. XLL involved in the antifungal bioassay; XBZ and YX partly involved in the synthesis of title compounds; GFX and XHZ partly involved in the synthesis of intermediates. All authors read and approved the final manuscript.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21272266).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional files
13065_2016_186_MOESM1_ESM.docx
Additional file 1. The experimental procedures of intermediates 3, 5, 6, 7, 8, 9 and title compounds 10, and the data of 1H NMR, 13C NMR, IR and HRMS or elemental analysis of target compounds 10.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lei, P., Zhang, X., Xu, Y. et al. Synthesis and fungicidal activity of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline. Chemistry Central Journal 10, 40 (2016). https://doi.org/10.1186/s13065-016-0186-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-016-0186-8