Abstract
Background
In Vietnam, malaria persists in remote forested regions where infections are spatially heterogeneous, mostly asymptomatic and with low parasite density. Previous studies in Vietnam have investigated broad behavioural concepts such as ‘engaging in forest activities’ as risk factors for malaria infection, which may not explain heterogeneity in malaria risk, especially in malaria elimination settings.
Methods
A mixed methods study combining ethnographic research and a cross-sectional survey was embedded in a 1-year malariometric cohort study in three ethnic minority villages in South Tra My district, Quang Nam Province in Central Vietnam. Qualitative data collection included in-depth interviews, informal conversations and participant observations over a 2-month period, and the findings were used to develop the questionnaire used in the cross-sectional survey. The latter collected data on evening activities, mobility patterns and household characteristics. The primary outcome, recent exposure to malaria, was defined using the classification and regression tree method to determine significant changes in antibody titres during the year preceding the survey. Risk factor analyses for recent exposure to malaria were conducted using logistic regression.
Results
22 in-depth interviews and numerous participant observations were recorded during the ethnographic research (April to June 2015), and 160 adults (86% response rate) responded to the cross-sectional survey (November to December 2015). Recent exposure to Plasmodium falciparum malaria was estimated at 22.9 and at 17.1% for Plasmodium vivax. Ongoing malaria transmission appears to be maintained by activities that delay or disrupt sleeping in a permanent structure in which a bed net could be hung, including evening drinking gatherings, fishing, logging in the forest and outdoor TV watching.
Conclusions
Vector control tools for outdoor evening activities in villages as well as at farms, forest and river locations should be incorporated into current malaria elimination efforts in Central Vietnam. Micro-epidemiology studies using mixed-methods designs can provide a comprehensive understanding of the malaria risk at fine spatial scales and better inform the implementation of targeted interventions for malaria elimination.
Similar content being viewed by others
Background
The burden of malaria in the Greater Mekong Subregion (GMS) has declined substantially over recent decades [1]. The Vietnamese government is aiming to achieve national malaria elimination by 2030 in accordance with the GMS regional malaria elimination strategy [1], buoyed by the success of an intensive malaria control programme that led to a more than 90% reduction in malaria cases since 2000 [2]. In Vietnam, malaria persists at low prevalence mainly in remote forested regions, many of which are inhabited by ethnic minority populations practicing subsistence slash and burn agriculture [3,4,5,6,7]. Within these persisting transmission foci, malaria is characterized by a high prevalence of asymptomatic infections with considerable fine-scale spatial heterogeneity, whereby malaria risk can vary substantially within and between villages [5, 8]. Determinants of fine-scale heterogeneity in malaria infection in low transmission settings are in general not well understood [9,10,11]. In settings approaching elimination, characterizing risk factors amongst sub-groups who continue to be at risk of malaria, despite overall declining incidence and implementation of malaria control measures, is crucial, as this small proportion of the population, often with asymptomatic infections, may serve as a reservoir of infections for whenever local conditions are permissive for malaria transmission [12, 13].
Studies from Central Vietnam have previously shown that age, bed net use and spending nights at farms and fields located in forested areas away from the village were important risk factors for malaria infection [4,5,6]. However, these risk factors were identified in studies that were conducted prior to the upscaling of LLIN distribution and awareness campaigns from 2010 onwards, which included promotion of LLIN use at forest farms as well as in villages. No recent studies have investigated socio-behavioural risk factors for malaria infection in Central Vietnam, which may have shifted since the onset of expanded malaria control activities. Furthermore, the primary vector in Central Vietnam, i.e. Anopheles dirus senso stricto demonstrates a preference for outdoor and early evening biting [14, 15], which implies that outdoor early evening activities may favour exposure to biting vectors that cannot be prevented by sleeping under LLINs at night, thus some risk factors relating to evening outdoor exposure may have been missed in previous studies.
Therefore, this study aimed to gain a detailed understanding of human behaviours during vector biting times that may increase the risk of malaria infection in a low-transmission setting, to inform further improvement of malaria elimination activities in this region.
Methods
Study setting and population
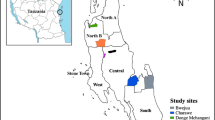
The study was based in Tra Cang Commune, in Nam Tra My district, Quang Nam Province, Central Vietnam. Tra Cang had a population of approximately 4000 people at the time of the study, almost all Xe Dang, an ethnic minority population mostly living in the central mountainous inland regions of Vietnam. Tra Cang commune comprises seven administratively-defined villages each comprising several hamlets of Xe Dang households. Most Xe Dang families also maintain a house or hut at their farms and rice fields, and reside there according to seasonal work requirements.
Both Plasmodium falciparum and Plasmodium vivax malaria transmission can occur year-round, with two peaks of transmission in June/July and October/November. Since 2005, malaria cases had declined substantially in Tra Cang, but a local outbreak occurred in 2012 and 2013, followed by a steady decrease since 2014 (Kattenberg et al., pers. comm.). Health care services in Tra Cang commune includes village health workers (one to two per village), a commune health centre (CHC) staffed by Xe Dang and Kinh (majority Vietnamese ethnic group) staff, and the district hospital, which is about half an hour by motorbike from the CHC. Malaria control activities include distribution of long-lasting insecticidal nets (LLINs), as well as provision of malaria testing (rapid diagnostic test and microscopy) and treatment (dihydroartemisinin-piperaquine and chloroquine were first-line treatment for P. falciparum and P. vivax, respectively, at the time of the study) at the CHC and at two village malaria posts in Village 5 and Village 7. Indoor residual spraying of houses had not been systematically undertaken at the time of the study, though public buildings such as schools were sprayed during the last outbreak in 2012/13. Intensive treatment-based control efforts were conducted between 2012 and 2014 in response to the outbreak, including treating entire households with the first-line treatment where one malaria-positive case was detected by rapid diagnostic test (RDT), and treating entire hamlets when RDT-positive cases were detected in two or more households (A. Erhart, pers. comm.).
Study design
A sequential mixed methods study [16], comprising a qualitative ethnographic strand (April to June 2015) followed by a cross-sectional survey (November to December 2015) was conducted ancillary to a cohort study (‘MAPARES study’), that followed the entire population of three hamlets (Tu Nak and Tak Lang in Village 5, Xe Xua in Village 7) through six consecutive malariometric surveys and passive case detection (PCD) between November 2014 and December 2015. In the cohort study, finger prick blood samples were collected and stored for light microscopy and PCR detection of Plasmodium spp. infection in all six surveys, as well as for serological analysis of antibody levels to two recombinant P. falciparum antigens (AMA1 [17] and GLURP-R2 [18]), and two recombinant P. vivax antigens (AMA1 [19] and MSP1–19 [20]) from the first and last survey. By the end of the cohort study, four asymptomatic infections had been confirmed by PCR while no cases were detected by PCD.
In the mixed methods study, the qualitative ethnographic strand aimed to explore the local context and identify possible risk factors for malaria that had not previously been identified, and the cross-sectional survey aimed to test whether these risk factors were associated with recent malaria infection.
Data collection and analysis
Data collection and analysis for the qualitative and quantitative strands are described separately below, and reported according to the STROBE statement for reporting observational epidemiological research [21], supplemented by COREQ guidelines for reporting qualitative research [22].
Qualitative strand
Research team
The field research team comprised the first author, the local principal investigator (NXX) and research assistants from the National Institute of Malariology, Parasitology and Entomology (NIMPE), Hanoi, previously trained in qualitative ethnographic research methods. The study team resided in Tra Cang for 2 months (April to June 2015) supported by the health staff at the Tra Cang CHC. The research team from NIMPE had extensive field experience in similar settings, and had previously visited the study setting on several occasions during the MAPARES study.
Participants and sampling
An open-ended research design centred on ethnographic methods guided the fieldwork. A purposive and adaptive sampling strategy was used to select informants, based on criteria such as history of malaria infection, mobility patterns, age, sex, and occupation. Emerging findings led to refinement of the sampling strategy and topic guides in order to maximize both variation and depth of information in the sample.
Data collection
Data collection methods included audio-recorded in-depth interviews, informal conversations (not audio-recorded but recalled and summarized in field notes), informal group discussions, and participant observation. Participants were usually first visited at their hamlet houses, but the study team also accompanied participants to their farms and fields, especially those who slept overnight in their plot huts. Interviews were also conducted in the general store, schools and CHC located in the administrative centre of Village 5. All interviews were conducted in Vietnamese, with local Xe Dang guides assisting as translators for some interviews. Field diaries and memos were maintained to aid data interpretation.
Data analysis
Data were intermittently analysed in the field concurrent with data collection, leading to refinement of topic guides and further sampling. At the end of the fieldwork period, all data were imported into NVivo (NVivo for Mac v11.40, QSR International Pty Ltd) for data management. Descriptive open coding and analysis focused on agricultural and social activities occurring at times and locations that may be associated with exposure to local vectors’ bites, but also describing the general socio-cultural context, health-seeking behaviour and local understandings of malaria.
Quantitative strand
Participants
A simple random sample of 186 individuals aged 16 years and over was drawn from the population census of two hamlets (Xe Xua and Tu Nak), where malaria infections had been detected during the MAPARES study. The sample size calculation was based on detecting, with 80% power and at 5% significance, a minimum 20% difference in the odds of recent malaria exposure (defined by serology), for individuals staying overnight at forest farms and fields, compared to individuals who do not. As malaria sero-prevalence was unknown at the time of the cross-sectional survey, the maximum sample size (n = 186 at 50% exposed-group prevalence) was used.
Data collection
The survey comprised a close-ended structured paper-based questionnaire. The questionnaire was piloted and revised, and the final version back-translated into English and checked for clarity. Trained surveyors from NIMPE administered the questionnaire at respondents’ households. Surveyors made up to three attempts to reach selected individuals. Data were double-entered and cleaned using Epi Info (Center for Disease Control and Prevention, Atlanta, USA).
Variables
The primary outcome was defined as recent exposure to malaria infection, estimated by serology and defined below. Serology was used because only four PCR-positive cases were detected during the six surveys, but also because serology outcomes may be more robust markers of exposure to malaria infection since it reflects cumulative changes in antibody titres over time, while PCR only detects malaria infections present at the time samples were taken [23,24,25,26,27,28]. Individual mean percent-positive antibody levels (mean ‘PP’; mean value among duplicates of the sample optical density (OD) measured as a proportion of the strong positive control OD) against two P. falciparum antigens (AMA1, GLURP2) and two P. vivax antigens (AMA1, MSP1) were defined for all participants in the first and last (sixth) MAPARES surveys. Subsequently, these continuous variables were categorized into four groups indicating the extent of seropositivity (strong positive, weak positive, grey (transition) zone, negative) using the classification and regression tree (CART) method [29] and implemented using CART ® software (Salford Systems, San Diego, USA). Cut-off points were defined using CART regression on each antibody distribution in the first survey, and applied to the sixth survey (Additional file 1). Regression trees were also computed for the changes in mean PPs from Survey 1 to Survey 6, to identify important minimum absolute increase and relative percent decrease that could be considered indicative of recent exposure and non-exposure, respectively. The following algorithm was used to define ‘recent exposure to malaria infection’:
-
Individual was negative or grey zone in Survey 1, and moved to a higher category (weak or strong positive) with a minimum absolute increase in PP in Survey 6, for one or more antibodies;
-
Individual was weak or strong positive in Survey 1, and had either a minimum absolute increase in PP in Survey 6, or less than the relative minimum decrease, for one or more antibodies;
-
Individual was strong positive in Survey 6 for one or more antibodies, if missing from Survey 1;
-
Individual was strong positive in Survey 1 for one or more antibodies, if missing from Survey 6.
Exposures
The research focused on identifying activities that may increase exposure to vector bites as risk factors for recent exposure to malaria infection. Based on the qualitative strand, the questionnaire included items about time spent in locations of presumed differential exposure to biting vectors (e.g. deep forest, forest farms and fields, river, hamlet), as well as evening and night time activities in these different locations that delay usual sleeping time and/or disrupt bed net use (e.g. drinking, outdoor TV watching, hunting, fishing). Several household-level variables were also included, such as household size [30], number of children, household ITN/LLIN coverage, housing construction, and presence of another recently exposed individual. The assumed causal relationships between these variables is depicted as a directed acyclic graph [31] in Fig. 1, and formed the basis of the modelling strategy.
Statistical analysis
Logistic regression models with robust standard errors to account for clustering by household were used to calculate crude and adjusted odds ratios for the association between exposure variables and recent malaria exposure, separately for P. falciparum and P. vivax. In line with the causal modelling approach, the emphasis was on appropriate adjustment for confounding rather than statistical significance alone, with the number of exposure variables balanced against data scarcity, which was assessed by inflation of standard errors upon inclusion of additional variables. All exposure variables with p values less than 0.2 in the unadjusted analyses were included in the multi-variable models. Given the small sample size relative to the number of exposure variables, individual-level variables with p value exceeding 0.2 were removed from the multi-variable models, before household-level variables were included. Household-level variables were then retained if their inclusion meaningfully shifted the effect estimates of exposure variables without substantially increasing standard errors. Age and sex were forced (confounding) variables in all models. Sub-group analyses were conducted for respondents who slept overnight at their forest farms and fields, as their overall exposure profile was considered to differ from respondents who always slept in their hamlet. Sensitivity analyses restricted to individuals with serological data in both Survey 1 and Survey 6 were run. All regression analyses were conducted in Stata/IC 13 (StataCorp LLC, College Station, Texas, USA).
Ethics
The study was approved by the institutional review boards of the Institute of Tropical Medicine, Antwerp, Belgium (ITM 1043/15), and the National Institute of Malariology, Parasitology and Entomology, Hanoi, Vietnam. Participants were included in the study if they were aged 16 years and over (the age of majority in Vietnam) and verbal consent was given. Ethical review and consent procedures were additional to those in the MAPARES study, which had separate approvals (ITM-UZA 958/14).
Results
Characteristics of study population
In the qualitative strand, 22 in-depth interviews were transcribed and translated, 10 informal conversations and numerous observations were recorded. Informants included health care workers, community leaders and officials, farmers, loggers and secondary school students. In the quantitative strand, 160 (86%) participated in the survey. All survey respondents were of Xe Dang ethnicity, 52% were males and 48% were females, with a mean age of 35 years. Most (92%) were farmers, and 62.5% had not been educated beyond primary school level (Table 1).
Serological data was available for 140 of the 160 cross-sectional survey participants, including 125 who participated in the first screening, 119 for the sixth screening, and 104 for both screenings. The proportion of respondents categorized as weak or strong positive declined overall between Survey 1 and Survey 6 for all antibodies (Additional file 2). There were 32 (22.9%) respondents whose P. falciparum antibody levels remained stable or increased between the two surveys, and 24 (17.1%) respondents with stable or increasing P. vivax antibody levels who met the criteria for ‘recent exposure to malaria infection’.
Risk factors for recent exposure to malaria infection
Hamlet-based activities
Access to a bed net in hamlet houses was reported to be very high (97.5%), and 75% reported always sleeping under a bed net (Table 1), however only 30% of households had a household bed net ratio of 1–2 people per bed net. Only 10 (6.25%) surveyed individuals reported sleeping in another house in the same hamlet, 8 of whom reported using bed nets when sleeping in a different house. Overall, neither individual bed net use when sleeping in the hamlet or bed net condition was associated with recent P. falciparum or P. vivax infection (Tables 2, 3).
Consumption of rice wine in the evenings in the hamlets was very common amongst men (84%) and common amongst women (44%), and 16% of men and 8% of women (12% overall) reported to drink every evening (Table 1). Rice wine was usually consumed outdoors in groups and had multiple functions, such as to aid sleep and soothe aches and pains after a day spent working in the fields, to facilitate social interactions (“first we invite each other for drinking, then we start talking”), transactionally (“paying for favours in wine”), and for a range of traditional ceremonies. Rice wine consumption delayed sleeping time; the median sleeping time was 8 pm if not drinking (IQR 7–9 pm), 9 pm after drinking (IQR 9–10 pm). Evening drinking hindered bed net use as, when asked generally, 88.5% of survey respondents reported to always sleep under a bed net, but only 65.4% reported to sleep under a bed net after drinking in the evening. There was a non-significant trend towards increased risk of P. falciparum exposure amongst daily drinkers in multivariable analyses (OR 2.15, 95% CI 0.71–6.51) (Table 4). However, when restricting analysis to individuals who never slept at the farms, daily drinking was their main risk factor for recent P. falciparum exposure (OR 9.52, 95% CI 1.93–46.87, p = 0.006) (Table 4).
Watching television/DVDs (hereafter ‘TV’) in the evenings was a common social activity. Most often, people watched TV together in a central house (26.5%), particularly in Tu Nak, many or all of whom sit outside as there is not enough space in the house. Only 17.5% watched TV at home (thus indoors), reflecting low TV ownership (Table 1). Watching TV in the evenings at another’s house, a proxy for outdoor TV watching, was associated with increased risk of recent P. vivax exposure (OR 6.29, 95% CI 1.49–26.58) (Table 5).
Forest farm and field-based activities
There were 72 (45%) survey respondents who slept overnight at their forest farms and fields. The longest duration of stay was mostly less than 1 week and was not associated with recent malaria, but 10% of overnight sleepers stayed for 1 week to more than 1 month continuously, which was associated with recent exposure to P. falciparum malaria amongst farm sleepers (adjusted OR 6.99, 95% CI 1.22–40.01, p = 0.029) (Table 4).
Over 90% of farm sleepers reported to sleep under a ITN or LLIN, and qualitative research suggested that bed net use at farms was consistently high owing to perceived high insect nuisance. Evening sleeping times at farms were earlier than in the hamlets (median sleep time 7 pm, IQR 6–8 pm). Not sleeping under a bed net at the farm was associated with recent P. vivax exposure (adjusted OR 17.57, 95% CI 1.91–160.80) (Table 5).
Evening drinking also occurs at the farms and fields (18.1% of men and 16.9% of women sometimes drink), but intermittently rather than regularly, and was not associated with P. falciparum or P. vivax exposure (Tables 2, 3).
Forest and river activities
46% of respondents reported spending evening hours in the forest, for collecting wild plant foods (24%), hunting (32.5%), collecting firewood (11.3%) and logging (3.8%) (Table 1). Different forest activities had different exposure patterns. Respondents reported to go hunting at night, but sleep at their plot hut or village house, whereas logging required multi-night stays in the forest. Logging was strongly associated with recent exposure, particularly for P. falciparum (OR 10.9, 95% CI 2.01–58.51, p = 0.006) but also for P. vivax (OR 5.45, 95% CI 1.26–22.76, p = 0.02). Insecticide-treated hammock nets were rarely taken to the forest (only two individuals in the survey).
Evening fishing was reported by 20 (12.50%) respondents, mostly men (75%) and mostly in Xe Xua hamlet where the river is located. Men went fishing in small groups of 4–6 people and returned late in the evening (median 10 pm, IQR 9 pm–12 am). Evening fishing was weakly associated with recent P. falciparum exposure amongst farm sleepers (OR 7.99, 95% CI 1.10–58.04, p = 0.04) (Table 4).
Demographic, household and other variables
Increasing age was associated with increased risk of recent exposure to P. falciparum, less so for P. vivax. Females were at higher risk of recent malaria exposure in univariable analyses, an effect that strengthened after adjusting for evening and outdoor activities, which were more prevalent in males.
Higher numbers of children living in a household was associated with decreased risk of P. falciparum and P. vivax exposure. Household bed net ratio or other seropositive members were not associated with P. falciparum or P. vivax exposure in adjusted analyses. Having a hamlet house built on stilts rather than ground level was not associated with P. falciparum or P. vivax exposure overall, but was protective against P. falciparum exposure amongst non-farm sleepers (OR 0.12, 95% CI 0.03–0.60, p = 0.01) (Table 4).
Sensitivity analyses
Restricting the analyses to only individuals with complete serology data (n = 104) led to minor fluctuations in effect sizes for most variables, except the effect of logging for P. falciparum exposure (OR attenuated from 10.9 to 3.3), but this may be affected by having only three loggers in the model with complete serology data. Participation in screening surveys was not associated with recent malaria exposure (Tables 2, 3). Different definitions for seropositivity were assessed, including defining a cut-off based on number of standard deviations above the negative control, or by applying a mixture model [32, 33], which in general resulted in a larger proportion of the population considered ‘seropositive’ and smaller effect sizes in risk factor analyses.
Discussion
This study used a mixed-methods study design to identify and further explain local risk factors for malaria infection in Central Vietnam. Evening outdoor activities such as drinking and TV watching were the main hamlet-based activities linked to recent P. falciparum and P. vivax exposure, respectively, but were not associated with malaria exposure amongst farm sleepers. Instead, farm sleepers, who spend variable proportions of time residing at their farms away from the hamlet throughout the year, were more likely to be recently exposed to P. falciparum malaria if they went fishing or logging in the evenings. At farms, TV watching was unavailable and drinking occurred much less frequently, whereas fishing and logging were more common amongst farm sleepers than non-farm sleepers. Apart from bed net use, no evening activities specifically conducted whilst staying overnight at forest farms and fields could be identified as risk factors.
The main findings of this study point to the importance of a micro-epidemiological approach in pre-elimination settings with shifting epidemiology. Broadly categorized behaviours previously recognized as risk factors, such as overnight sleeping at forest farms and bed net use in the village [4,5,6], were not associated with recent malaria exposure in this adult study population in a low prevalence setting. Instead, ongoing malaria transmission appears to be maintained by activities that delay or disrupt sleeping in a permanent structure in which a bed net could be hung, including evening drinking gatherings, fishing, logging in the forest and outdoor TV watching. Additionally, no bed net use at forest farms remained a risk factor for recent P. vivax exposure. Thus, this study provides detailed information on human behaviours that contribute to residual transmission, in contrast to previous literature in which residual transmission has been largely defined and characterized with respect to vector behaviour [34, 35] with comparatively limited consideration of human behaviours that maintain residual transmission (exceptions include [36, 37]).
Females were more likely to be classified as recently exposed by serology, and the effect of female sex became more pronounced after adjustment for behavioural-related risk factors, a finding that is in contrast to previous studies in this area that found males to be at higher risk of malaria when measured by PCR or microscopy rather than serology [4, 6, 38]. In the full MAPARES cohort, though numbers were small, descriptively an age-dependent sex difference in seropositivity was observed: there was no sex difference in seropositivity up to 10 years of age or after 50 years of age, but higher proportions of seropositive women aged 10–49. This might be explained by estrogen-mediated sex differences in immune response to parasitic infections, including Plasmodium parasites [39,40,41,42,43]. It is also possible that the analyses were confounded by unknown exposure-related behaviours that were more prevalent amongst females than males. Previous studies that have found higher seropositivity rates in females to have not distinguished intrinsic biological factors versus risk behaviours as determinants [23, 25, 44]. The association between sex and seropositivity is inconsistent in the literature (e.g., males at higher risk in [45], no difference in [46, 47]), and may vary by antibody [23, 25] as well as by study setting. More detailed exploration of sex differences in malaria seropositivity outcomes should be considered in future studies.
Limitations
Though serological markers can be used to describe fine-scale heterogeneity in malaria infection in both high and low transmission settings [24, 27, 48, 49], it nonetheless remains difficult to define serological outcomes that accurately reflect recent malaria infection using a limited set of antibodies [50]. Specificity was favoured over sensitivity in selecting the algorithm used to generate the seropositivity cut-points, in order to define the primary outcome of ‘recent malaria exposure’. Alternative definitions of seropositivity were considered, but it was considered incongruous to use cut-offs that defined a larger proportion of the study population as recently exposed, given there were so few current infections detected during the cohort study period. Nonetheless, the specific definition used may have excluded some recently exposed individuals with attenuated antibody responses. The analyses for P. falciparum are considered more reliable than for P. vivax to detect risk factors for recent exposure to new infections as the latter could not be separated from recrudescence for P. vivax.
This study was limited by the sample size, which in the cross-sectional survey was not sufficiently powered for the various sub-group analyses, and complete serological data was only available for 104 of 160 (65%) respondents, reflecting incomplete participation in the cohort study wherein complete serological data was only available for 58.6% of respondents. The sampling frame was restricted to adults as it was anticipated that there would be a high non-response rate from children requiring parental consent to participate as many children reside at school during the week and then accompany their parents to the farms on the weekend. Nonetheless, this restricts the generalizability of findings from this study, including no association between hamlet bed net use and recent malaria exposure, to adult populations in this setting only. Furthermore, a complete net integrity assessment was not undertaken, which restricts the validity of the bed net condition variable. The proportion considered recently exposed in this analysis was higher than in the MAPARES cohort overall (10.4% for P. falciparum and 12.3% for P. vivax), largely reflecting the exclusion of children in the cross-sectional survey, who had lower malaria antibody levels (Kattenberg et al., pers. comm.).
Beyond these sample limitations, in general there are challenges in conducting studies in low prevalence settings, especially when risk activities are practiced by a small proportion of the population (e.g. logging in the forest) that limit statistical power to detect important risk factors and calculate meaningful confidence intervals. Triangulating quantitative against qualitative findings partly ameliorates this challenge, as in-depth interviews and observation of the setting provides additional context that can support or refute the quantitative results.
Implications and further research
As residual transmission appears to be maintained by outdoor evening activities that disrupt or delay sleeping, long-lasting insecticidal hammock nets may be of limited utility for achieving further reductions in malaria transmission, despite being designed for similar forest malaria settings in Vietnam [51]. Declining malaria incidence in Tra Cang in recent years demonstrates that malaria can be very well controlled with existing interventions, which have combined LLIN distributions, ensuring access to diagnosis and treatment, and active case detection and treatment campaigns despite ongoing structural vulnerability to malaria. In addition to these malaria control activities, there may be a role for supplementary vector control tools such as personal topical repellents for use at farms and in the forest, or spatial repellents for evening gatherings in hamlets, though neither tool shows consistent efficacy [52, 53], and effectiveness is dependent on acceptance and sufficient use by the target population [54].
Conclusions
Persistent malaria transmission in Tra Cang, Central Vietnam, appears to be maintained by outdoor evening activities that delay or disrupt sleeping in a permanent structure in which a bed net could be hung, including evening drinking gatherings, fishing, logging in the forest and outdoor TV watching, as well as non-use of bed nets at forest farms and fields. In addition to existing malaria control efforts, vector control tools adapted for use during outdoor evening activities in villages as well as at farms, forest and river locations should be incorporated into malaria elimination efforts in Central Vietnam. Effectively targeting residual malaria transmission remains an area of active research, to which studies using mixed-methods designs can contribute by identifying context-specific determinants of persisting malaria risk, which may guide further research and application of appropriate interventions in settings in which residual transmission occurs.
References
WHO. Strategy for malaria elimination in the Greater Mekong Subregion: 2015–2030. Manila: World Health Organization Regional Office for the Western Pacific; 2015.
WHO. World malaria report 2015. Geneva: World Health Organization; 2016.
Erhart A, Thang ND, Hung NQ, Toi LV, Hung LX, Tuy TQ, et al. Forest malaria in Vietnam: a challenge for control. Am J Trop Med Hyg. 2004;70:110–8.
Erhart A, Thang N, Van Ky P, Tinh T, Van Overmeir C, Speybroeck N, et al. Epidemiology of forest malaria in Central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4:58.
Thanh PV, Van Hong N, Van Van N, Van Malderen C, Obsomer V, Rosanas-Urgell A, et al. Epidemiology of forest malaria in Central Vietnam: the hidden parasite reservoir. Malar J. 2015;14:86.
Thang N, Erhart A, Speybroeck N, Hung L, Thuan L, Hung C, et al. Malaria in Central Vietnam: analysis of risk factors by multivariate analysis and classification tree models. Malar J. 2008;7:28.
Hung LQ, Vries PJd, Giao PT, Nam NV, Binh TQ, Chong M, et al. Control of malaria: a successful experience from Viet Nam. Bull World Health Organ. 2002;80:660–6.
Van Nguyen H, van den Eede P, van Overmeir C, Thang ND, Hung LX, D’Alessandro U, et al. Marked age-dependent prevalence of symptomatic and patent infections and complexity of distribution of human Plasmodium species in Central Vietnam. Am J Trop Med Hyg. 2012;87:989–95.
Cui L, Yan G, Sattabongkot J, Chen B, Cao Y, Fan Q, et al. Challenges and prospects for malaria elimination in the Greater Mekong Subregion. Acta Trop. 2012;121:240–5.
Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–39.
Bannister-Tyrrell M, Verdonck K, Hausmann-Muela S, Gryseels C, Ribera JM, Grietens KP. Defining micro-epidemiology for malaria elimination: systematic review and meta-analysis. Malar J. 2017;16:164.
Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–40.
Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165.
Van Bortel W, Trung H, Hoi L, Van Ham N, Van Chut N, Luu N, et al. Malaria transmission and vector behaviour in a forested malaria focus in Central Vietnam and the implications for vector control. Malar J. 2010;9:373.
Obsomer V, Defourny P, Coosemans M. The Anopheles dirus complex: spatial distribution and environmental drivers. Malar J. 2007;6:26.
Tashakkori A, Teddlie C. Mixed methodology: combining qualitative and quantitative approaches. Thousand Oaks: Sage Publications; 1998.
Kocken CH, Withers-Martinez C, Dubbeld MA, van der Wel A, Hackett F, Valderrama A, et al. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect Immun. 2002;70:4471–6.
Theisen M, Soe S, Oeuvray C, Thomas AW, Vuust J, Danielsen S, et al. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun. 1998;66:11–7.
Kocken CH, Dubbeld MA, Van Der Wel A, Pronk JT, Waters AP, Langermans JA, et al. High-level expression of Plasmodium vivax apical membrane antigen 1 (AMA-1) in Pichia pastoris: strong immunogenicity in Macaca mulatta immunized with P. vivax AMA-1 and adjuvant SBAS2. Infect Immun. 1999;67:43–9.
Soares IS, da Cunha MG, Silva MN, Souza JM, Del Portillo HA, Rodrigues MM. Longevity of naturally acquired antibody responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1. Am J Trop Med Hyg. 1999;60:357–63.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57.
Wong J, Hamel MJ, Drakeley CJ, Kariuki S, Shi YP, Lal AA. Serological markers for monitoring historical changes in malaria transmission intensity in a highly endemic region of Western Kenya, 1994–2009. Malar J. 2014;13:451.
van den Hoogen LL, Griffin JT, Cook J, Sepúlveda N, Corran P, Conway DJ, et al. Serology describes a profile of declining malaria transmission in Farafenni, The Gambia. Malar J. 2015;14:416.
Kerkhof K, Sluydts V, Willen L, Kim S, Canier L, Heng S, et al. Serological markers to measure recent changes in malaria at population level in Cambodia. Malar J. 2016;15:529.
Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SLR, Carneiro I, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–13.
Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, et al. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201:1764–74.
Bejon P, Turner L, Lavstsen T, Cham G, Olotu A, Drakeley CJ, et al. Serological evidence of discrete spatial clusters of Plasmodium falciparum parasites. PLoS ONE. 2011;6:e21711.
Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and regression trees. Boca Raton: CRC Press; 1984.
Kaindoa EW, Mkandawile G, Ligamba G, Kelly-Hope LA, Okumu FO. Correlations between household occupancy and malaria vector biting risk in rural Tanzanian villages: implications for high-resolution spatial targeting of control interventions. Malar J. 2016;15:199.
Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48.
Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, et al. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195.
Cook J, Speybroeck N, Sochanta T, Somony H, Sokny M, Claes F, et al. Sero-epidemiological evaluation of changes in Plasmodium falciparum and Plasmodium vivax transmission patterns over the rainy season in Cambodia. Malar J. 2012;11:86.
Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes—New insights into malaria vectors. Ryeka: InTech; 2013.
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330.
Gryseels C, Durnez L, Gerrets R, Uk S, Suon S, Set S, et al. Re-imagining malaria: heterogeneity of human and mosquito behaviour in relation to residual malaria transmission in Cambodia. Malar J. 2015;14:165.
Monroe A, Asamoah O, Lam Y, Koenker H, Psychas P, Lynch M, et al. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention. Malar J. 2015;14:35.
Imwong M, Nguyen TN, Tripura R, Peto TJ, Lee SJ, Lwin KM, et al. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand–Myanmar border areas, Cambodia, and Vietnam. Malar J. 2015;14:381.
Cernetich A, Garver LS, Jedlicka AE, Klein PW, Kumar N, Scott AL, et al. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect Immun. 2006;74:3190–203.
Klein PW, Easterbrook JD, Lalime EN, Klein SL. Estrogen and progesterone affect responses to malaria infection in female C57BL/6 mice. Gend Med. 2008;5:423–33.
Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44.
Guerra-Silveira F, Abad-Franch F. Sex Bias in infectious disease epidemiology: patterns and processes. PLoS ONE. 2013;8:e62390.
Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta-analysis. Biol Rev Camb Philos Soc. 2017;92:551–71.
Biggs J, Raman J, Cook J, Hlongwana K, Drakeley C, Morris N, et al. Serology reveals heterogeneity of Plasmodium falciparum transmission in northeastern South Africa: implications for malaria elimination. Malar J. 2017;16:48.
Ghinai I, Cook J, Hla TTW, Htet HMT, Hall T, Lubis IN, et al. Malaria epidemiology in central Myanmar: identification of a multi-species asymptomatic reservoir of infection. Malar J. 2017;16:16.
Cook J, Reid H, Iavro J, Kuwahata M, Taleo G, Clements A, et al. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J. 2010;9:169.
Yalew WG, Pal S, Bansil P, Dabbs R, Tetteh K, Guinovart C, et al. Current and cumulative malaria infections in a setting embarking on elimination: Amhara, Ethiopia. Malar J. 2017;16:242.
Mosha JF, Sturrock HJ, Greenwood B, Sutherland CJ, Gadalla NB, Atwal S, et al. Hot spot or not: a comparison of spatial statistical methods to predict prospective malaria infections. Malar J. 2014;13:53.
Stevenson JC, Stresman GH, Baidjoe A, Okoth A, Oriango R, Owaga C, et al. Use of different transmission metrics to describe malaria epidemiology in the highlands of western Kenya. Malar J. 2015;14:418.
Helb DA, Tetteh KKA, Felgner PL, Skinner J, Hubbard A, Arinaitwe E, et al. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci USA. 2015;112:E4438–47.
Thang ND, Erhart A, Speybroeck N, Xa NX, Thanh NN, Ky PV, et al. Long-lasting insecticidal hammocks for controlling forest malaria: a community-based trial in a rural area of Central Vietnam. PLoS ONE. 2009;4:e7369.
Wilson AL, Chen-Hussey V, Logan JG, Lindsay SW. Are topical insect repellents effective against malaria in endemic populations? A systematic review and meta-analysis. Malar J. 2014;13:446.
Achee NL, Bangs MJ, Farlow R, Killeen GF, Lindsay S, Logan JG, et al. Spatial repellents: from discovery and development to evidence-based validation. Malar J. 2012;11:164.
Gryseels C, Uk S, Sluydts V, Durnez L, Phoeuk P, Suon S, et al. Factors influencing the use of topical repellents: implications for the effectiveness of malaria elimination strategies. Sci Rep. 2015;5:16847.
Authors’ contributions
MBT designed the study, conducted field data collection, analysed the data, and wrote the manuscript. NXX conceived of and designed the study, conducted field data collection, and critically revised the manuscript. JK analysed the serological data and critically revised the manuscript. NVV conceived of the study, oversaw data collection and revised the manuscript. VKAD collected data, aided in data interpretation. TMH, NVH, ERV, NTT, TTD contributed to the collection and analysis of serological data and revised the manuscript. ARU and KPG conceived of the study and revised the manuscript. AE conceived of the study, analysed the data and critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully acknowledge the support of the Tra Cang commune health centre staff and local community leaders for hosting the study team, assisting with logistics, acting as local village guides and Xe Dang interpreters as required. The authors also greatly appreciate the efforts of the data collectors and transcribers from the National Institute of Malariology, Parasitology and Entomology, and from Quang Nam provincial health department. We wish to thank the people who participated in the study, and the support and collaboration of the residents of Tra Cang commune. We also gratefully acknowledge the support of health staff at district, province and national level.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the confidential nature of the collected personal data but are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the institutional review boards of the Institute of Tropical Medicine, Antwerp, Belgium (ITM 1043/15), and the National Institute of Malariology, Parasitology and Entomology, Hanoi, Vietnam. Participants were included in the study only if they were aged 16 years and over (the age of majority in Vietnam) and verbal consent was given after study information forms that included details about the study and its benefits and potential risks were given or read to the participants. This consent process was additional to the requirements for participation in the MAPARES cohort study, which received separate approvals (ITM-UZA 958/14).
Funding
This study funded by The Belgian Development Cooperation (DGD-ITM Third Framework Agreement, 2014–2016) and The Global Health Group at the University of California, San Francisco (UCSF).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1.
Mean percent-positivity (PP) cut-points used to define seropositivity categories in 1st and 6th survey, and minimum changes in mean PPs between surveys used to define stable or increased antibody level.
Additional file 2.
Seropositivity categories at first and sixth screening survey, by antibody.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bannister-Tyrrell, M., Xa, N.X., Kattenberg, J.H. et al. Micro-epidemiology of malaria in an elimination setting in Central Vietnam. Malar J 17, 119 (2018). https://doi.org/10.1186/s12936-018-2262-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-018-2262-0