Abstract
Background
Qianggu Capsule, a Chinese patent medicine, has been widely applied in the clinical practice of primary osteoporosis (POP) in recent years. This study aims to summarize the effectiveness and safety of Qianggu Capsule in treating POP.
Methods
We searched seven electronic databases, all searches ended in 30 September, 2015. All randomised controlled trials comparing the efficacy of Qianggu Capsule treatment with no treatment, placebo or conventional therapy for POP were included. Combined therapies of Qianggu Capsule were also included. Cochrane risk of bias tool was used to assess methodological quality of primary studies. Revman 5.2.0 software was used for data analysis.
Results
Ten trials were enrolled. The combined effect showed that Qianggu Capsule plus Caltrate D was better than Caltrate D on lumbar spine bone mineral density (BMD) (MD = 0.05 g/cm2; 95% CI: 0.02–0.07; P = 0.0004), femoral neck BMD (MD = 0.03 g/cm2; 95% CI: 0.01–0.05; P = 0.001), femoral great trochanter BMD (MD = 0.04 g/cm2; 95% CI: 0.03–0.06; P < 0.001). Meta-analysis exhibited a significant antiosteoporosis effect of Qianggu Capsule on femoral neck BMD (MD = 0.03 g/cm2; 95% CI: 0.01–0.05; P = 0.003) and femoral trochanteric BMD (MD = 0.07 g/cm2; 95% CI: 0.02–0.12; P = 0.006) compared with α-D3 capsule. However, the methodological quality of included studies was low. Constipation and dry mouth were the most common adverse drug reactions of Qianggu Capsule. Finally the evidence level was evaluated to be low or very low.
Conclusions
The effect of Qianggu Capsule for POP was supported in improving BMD. Due to the methodological drawbacks of the included studies, the conclusions should be treated with caution for future research.
Similar content being viewed by others
Background
Primary osteoporosis (POP) is one of the most common chronic conditions, and affects both old men and postmenopausal women [1, 2]. Osteoporosis is estimated to cause 1.5 million fractures every year in the United States [3]. In China, there have been about 202.43 million people aged 60 years and older at the end of 2013, which faces higher risk of osteoporosis-related fractures [4]. From 2002 to 2006, the rates of hip fracture over age 50 years have increased 58% in women and 49% in men based on a population-based study in Beijing [5]. Most important of all, the most serious consequences of osteoporotic fractures, especially hip fracture, are the increasing proportion of mortality and disability [6]. Therefore, interventions to treat POP or prevent osteoporotic fractures should be implemented. Although research efforts have been expanded for several decades, an urgent need exists for continued improvement so far, particularly in the treatment of POP.
Many strategies are available to treat POP, but pharmacological treatments still plays the dominant role. Major antiosteoporosis agents including bisphosphonates, denosumab, hormone replacement therapy, selective estrogen receptor modulators, recombinant human parathyroid hormone and strontium ranelate are currently available on the market [7]. The common outcomes are osteoporotic fractures [8, 9], bone mineral density (BMD) value [10], bone turnover markers [11], pain assessment [12], quality of life [13], and adverse event or adverse drug reaction mainly from antiosteoporosis drugs [14]. In some cases, POP patients can benefit from drug therapy optimization and combination therapy. Despite the fact that several western medicines have demonstrated to be effective in the treatment of POP, however, poor medication adherence remains a major problem [15, 16]. Suboptimal adherence to therapy may partially be due to adverse effects of long-term conventional antiosteoporosis drugs, such as bisphosphonates [17, 18]. Hence, there is a requirement for long-term treatment to be associated with a positive benefit-risk balance [19]. Now more and more studies of complementary and alternative medicine have increased the awareness of the problem and have improved our understanding of the prevention and control of osteoporosis. In China, herbal fufang and single Chinese herb have been widely used for the treatment of POP [20–22].
Qianggu Capsule, the main effective components of which are the total flavonoids of Rhizoma Drynariae (Gusuibu) [23], has been approved by China Food and Drug Administration for treating POP (drug approval numbers: Z20030007). According to the theory of traditional Chinese medicine and results of population pharmacokinetics, Qianggu Capsule has the effect of replenishing the kidney and strengthening the bones which applies to shen-yang deficiency pattern [24, 25]. Modern research has also proven that Qianggu Capsule can increase lumbar and femoral BMD, raise serum calcium, improve analgesia action, control the levels of serum IL-6 and TNFa, and accelerate the secretion of IL-4 in rats. No abnormal changes are found in the toxicity test [26]. So Qianggu Capsule is reliable and safe in laboratory studies.
In contrast to the wealth of data about the efficacy of chemical agents in the management of POP, information regarding their efficacy and safety in Chinese herbal medicine is relatively limited. In recent years, a large number of clinical studies reported the effect of Qianggu Capsule and Qianggu Capsule combined with antiosteoporosis drugs. Therefore, this systematic review provides an evidence of Qianggu Capsule for the management of POP from the randomised controlled trials.
Methods
The study protocol was previously registered in PROSPERO platform which could be available on https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015025784.
Data sources and searches
Seven electronic databases were searched from their inception until 30 September, 2015: PubMed, Cochrane CENTRAL, EMBASE, Chinese National Knowledge Infrastructure (CNKI), Wanfang database, Chinese Scientific Journals Database (VIP), Chinese Biomedical Literature Database (CBM). Additional published or unpublished literature was retrieved through manual searches of reference lists of included studies and key review articles, and from the files of content experts.
The search terms included “osteoporosis”, “primary osteoporosis”, “senile osteoporosis”, “postmenopausal osteoporosis”, “qianggu capsule”, “qiang gu capsule” and “Gusuibu”. Search terms used for PubMed were as follows: (osteoporosis OR primary osteoporosis OR senile osteoporosis OR postmenopausal osteoporosis) AND (qianggu capsule OR qiang gu capsule OR Gusuibu).
Types of studies
All completed randomised controlled trials comparing the efficacy of Qianggu Capsule treatment for POP were enrolled. Animal experiments were not inclusive.
Types of participants
The clinical diagnosis was required to be in accordance with the criteria of POP. It should be noted that some minor differences existed among different diagnostic criteria. For example, World Health Organization criteria (BMD of subjects, 2.5 SD [T-score < or = −2.5] lower than young adult mean value) [27] had a different numerical standard than that for Chinese criteria (BMD of subjects, 2 SD [T-score < or = −2] or less than 75% of lower than young adult mean value) [28, 29]. Generally, study population was mainly from middle-aged and aged people (≥40 years).
Types of interventions
In this review, randomised controlled trials that assessed the therapeutic effect of Qianggu Capsule, compared with no treatment, placebo or conventional therapy were considered. Combined therapies of Qianggu Capsule and other conventional interventions compared with other conventional interventions in randomised controlled trials were also enrolled. The interventions containing other complementary and alternative treatments (Chinese medicine, acupuncture, moxibustion, massage, yoga, tai chi, qigong, baduanjin, wuqinxi and so on) in the Qianggu Capsule or comparison group were excluded. The duration of treatment was required to be at least 3 months.
Types of outcomes
The primary outcome was osteoporosis-related fractures. The secondary outcomes analyzed in this review were BMD values, pain scores, quality of life, biochemical markers of bone turnover, and adverse event or adverse drug reaction (ADR).
Study selection
Two reviewers independently searched and screened the studies. Exclusion criteria included: (1) inappropriate study design, such as reviews, case reports, comments, letters; (2) duplicate trials; (3) not population of interest; (4) no Qianggu Capsule intervention; (5) lack of the above outcomes. After removing excluded abstracts, full articles were obtained and studies were screened again more thoroughly using the same exclusion criteria. Any disagreements were resolved through discussion with a third reviewer.
Data abstraction
Data abstraction was independently performed by two reviewers based on pre-piloted forms. A neutral third reviewer was consulted if there are still disagreements after discussion. The first author names and year of publication, sample size, diagnostic criteria, population characteristics (age and sex), duration of symptom, intervention details (medication doses, therapeutic regimen and treatment duration), and outcome data were extracted.
Risk of bias assessment in individual studies
We used the Cochrane risk of bias tool to assess methodological quality of included studies [30]. And two authors compared the evaluation results and discussed difference until agreement was reached. Selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias were evaluated respectively. The quality of included trials was divided into three levels: low risk of bias (all the items were in low risk of bias), high risk of bias (at least one item was in high risk of bias), unclear risk of bias (at least one item was in unclear).
Analytical approach
Data analysis was performed with Review Manager 5.2.0 software. Based on the continuous data, mean difference (MD) was used to assess the difference between experimental group and control group. Standardized mean difference (SMD) was considered if clinical outcome was the same but measured using different scales in the different trials. Risk ratio (RR) was used for the binary data. And the 95% confidence intervals (CI) were calculated in the meta-analysis. In a three-group design study that had two treatment groups of Qianggu Capsule and Qianggu Capsule plus antiosteoporosis drugs, the two comparisons were split in the meta-analysis. Heterogeneity was assessed by means of I2 statistic. If the I2 statistic indicated considerable heterogeneity (≥50%), we combined the summary measures across the studies using a random effects model that assumed that the included studies represent a sample from a larger population of studies [31]. Analysis of subgroups will be used if there are sufficient clinical trials for the same outcome.
Qualitative analysis of trial results
We evaluated the quality of the body of evidence adopting the GRADE approach [32, 33]. High quality evidence was considered as randomised controlled trials with low risk of bias that produced consistent, direct and precise results for the clinical outcome [34]. Three domains, including large magnitude of effect, all plausible confounding which can increase confidence in estimated effects, high dose–response gradient may increase the quality of evidence [35, 36]. Levels of quality of evidence were defined as high, moderate, low, very low [37].
Results
Characteristics of the studies
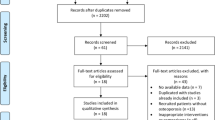
The search strategy identified 332 reports. After removal of duplicates, 220 records remained. After going through the titles and abstracts, 192 reports were excluded with at least one of following reasons: (1) animal experiments; (2) traditional review or not from POP patients; (3) lack of control group. Then the remaining 28 papers were further assessed with accessible full text. Eventually 10 reports [38–47] met the inclusion criteria for the review and 18 papers were excluded. The reasons for exclusion were: non-RCTs (n = 8), inappropriate intervention (n = 10). The screening process was showed in a PRISMA 2009 flow diagram (Fig. 1). All the studies were published in Chinese journals (from 2004 to 2013).
Of the 10 articles, 806 participants were enrolled in the review and depicted in Table 1. Eight trials used Chinese osteoporosis diagnostic criteria [38, 40–42, 44–47]. Two trials were also included because BMD was used for the diagnosis and evaluation [39, 43]. The average age ranged from 57.9 to 70.4 years. Course of disease was provided in only 2 trials [41, 47] and was not found in the remaining included studies.
To reduce the clinical heterogeneity among the studies, the interventions could be divided into 7 different subgroups as follows: (1) Qianggu Capsule versus Calcium gluconate [38]; (2) Qianggu Capsule versus Livial [39]; (3) Qianggu Capsule plus Caltrate D versus Caltrate D [40, 47]; (4) Qianggu Capsule versus Vitamin D2 and calcium hydrogen phosphate tablets [41]; (5) Qianggu Capsule versus α-D3 capsule [42, 43, 45]; (6) Qianggu Capsule and Calcium tablet versus Qianggu Capsule placebo and Calcium tablet [44]; (7) Qianggu Capsule plus Alendronate versus Alendronate [46]. The duration of treatment was not beyond 12 months.
All the studies reported different parts of BMD values [38–47]. Three studies used bone biochemical markers as surrogate outcome [42–44]. Seven studies reported adverse drug reaction (ADR) [38–40, 42, 43, 45, 46]. In addition, osteoporotic fractures, internationally recognized pain scales and quality of life were not evaluated in all trials.
Quality of methodological reporting
The methodological quality of primary studies was evaluated as low (as shown in Table 2). Only 1 trial reported random number table as the method of randomization [46]. A randomized, double-blind and placebo-controlled trial was identified [44]. Allocation concealment, blinding of participants and personnel were not found in the other studies. The blinding of outcome assessment was not stated in all trials. Two trials did not provide any information about the drop-outs or withdrawals [40, 44]. None of the trials registered or published the study protocol. So the selective reporting was unclear. Additionally, other sources of bias were identified as unclear in 3 trials because the baseline of the trials was not mentioned [38, 41, 44].
Effect of the interventions
All the included studies compared Qianggu Capsule practised alone or combined with antiosteoporosis drugs. According to the different intervention and control program, the interventions could be divided into the following subgroups.
1. Qianggu Capsule versus Calcium gluconate: there was a statistically significant difference between the groups in mean improvement on lumbar BMD favoring Qianggu Capsule intervention after 3 months (P < 0.05) [38].
2. Qianggu Capsule versus Livial: BMD in lumbar spine and femoral neck increased markedly in livial group, but statistical significance was not found in both groups after 6 months (P > 0.05) [39].
3. Qianggu Capsule plus Caltrate D versus Caltrate D: The combined analysis of two trials found a significant effect of Qianggu Capsule plus Caltrate D on lumbar spine BMD (MD = 0.05 g/cm2; 95% CI: 0.02–0.07; P = 0.0004, Fig. 2), femoral neck BMD (MD = 0.03 g/cm2; 95% CI: 0.01–0.05; P = 0.001, Fig. 3), femoral great trochanter BMD (MD = 0.04 g/cm2; 95% CI: 0.03–0.06; P < 0.001, Fig. 4) [40, 47]. In addition, there was no significant difference on ward’s BMD between the groups in the result of Xia et al. (P < 0.05) [40].
4. Qianggu Capsule versus Vitamin D2 and calcium hydrogen phosphate tablets: Qianggu Capsule group demonstrated a significant improvement on BMD of ulna and radius compared with Vitamin D2 and calcium hydrogen phosphate tablets group after 3 months (P < 0.05) [41].
5. Qianggu Capsule versus α-D3 capsule: There was no significant difference on lumbar spine BMD (MD = 0.05 g/cm2; 95% CI: −0.01–0.11; P = 0.09, Fig. 5) between the groups [42, 43, 45]. Meta-analysis indicated a significant antiosteoporosis effect of Qianggu Capsule on femoral neck BMD (MD = 0.03 g/cm2; 95% CI: 0.01–0.05; P = 0.003, Fig. 6) [42, 43, 45], femoral trochanteric BMD (MD = 0.07 g/cm2; 95% CI: 0.02–0.12; P = 0.006, Fig. 7) compared withα-D3 capsule [42, 45]. A remarkable improvement in ward’s BMD with Qianggu Capsule was identified in Gao’s study (P < 0.01) [45].
Meta-analysis of two trials showed that there was no difference in improving the level of calcium (MD = 0.01 mmol/L; 95% CI: −0.04–0.06; P = 0.69), phosphorus (MD = 0.01 mmol/L; 95% CI: −0.04–0.06; P = 0.67) and alkaline phosphatase (MD = 3.05 U/L; 95% CI: −4.66–10.76; P = 0.44) [42, 43]. In Wang’s study, Qianggu Capsule was better thanα-D3 capsule in lowering NTX/Cr (P < 0.01) [43].
6. Qianggu Capsule and Calcium tablet versus Qianggu Capsule placebo and Calcium tablet: Based on Calcium tablet as basic treatment, Qianggu Capsule was better than placebo in improving lumbar BMD value after 6 months (P < 0.01, P < 0.05). Qianggu Capsule plus Calcium tablet also significantly increased the level of bone gla protein, calcitonin and estradiol in the blood (P < 0.01); on the other, the excretion of urinary hydroxyproline and the level of parathyroid hormone was reduced (P < 0.01) [44].
7. Qianggu Capsule plus Alendronate versus Alendronate: The BMD difference of lumbar spine and wards area in combination therapy group was higher than Alendronate group after 6 months (P < 0.01) [46].
Adverse effects of Qianggu Capsule
Six trials reported ADRs of Qianggu Capsule used alone [38–40, 42, 43, 45]. Three patients (3/41, 7.32%) with constipation [38] and 2 patients (2/32, 6.25%) with mild constipation [42] were found in Qianggu Capsule group. Zhao et al. reported that 3 patients (3/34, 8.82%) with constipation were identified in Qianggu Capsule group, whereas 3 patients (3/35, 8.57%) with uncomfortable hepatic region, 2 patients (2/35, 5.71%) with cutaneous pruritus, and 3 patients (2/35, 5.71%) with colporrhagia in livial group [39]. Xia et al. found that 2 patients (2/29, 6.90%) with constipation or dry mouth in Qianggu Capsule group, 1 patient (1/29, 3.45%) with constipation in the control group [40]. Similarly, Wang et al. reported 2 cases (2/28, 7.14%) with constipation and 1 case (1/28, 3.57%) with dry mouth in Qianggu Capsule group [43]. The study conducted by Gao et al. showed that 12 cases (12/64, 18.75%) with mild constipation, 15 cases (15/64, 23.44%) with dry mouth, and 18 cases (18/64, 28.13%) with lower rhythm of the heart in Qianggu Capsule group, while 9 cases (9/64, 14.06%) with loss of appetite, headache, vomit and 6 cases with higher blood calcium levels in the control group [45]. Only 1 trial observed the ADR of combination therapy [46]. The result demonstrated that 6 cases (6/40, 15%) with nausea in combination therapy group and 3 cases (3/40, 7.5%) with nausea in Alendronate group.
All of the ADRs were not severe and relieved without any treatment. Constipation and dry mouth were the most common ADRs in the usage of Qianggu Capsule.
Quality of evidence
Based on the GRADE approach, low quality evidence (two trials, 208 participants) supported the Qianggu Capsule plus Caltrate D in improving BMD compared with Caltrate D; very quality evidence (three trials, 244 participants) supported the Qianggu Capsule in improving BMD compared withα-D3 capsule.
Discussion
Summary of the systematic review
More and more Chinese herbs have been historically used to treat bone metabolic diseases and known for anti-osteoporotic drugs [48–51]. The anti-osteoporosis effect of Rhizoma Drynariae and its extracts have attracted world-wide attention [52, 53]. Our systematic review is to assess the efficacy and safety of Qianggu Capsule (Rhizoma Drynariae) in osteoporosis therapy. The results of meta-analysis suggested that Qianggu Capsule plus Caltrate D was more effective than Caltrate D alone on lumbar spine, femoral neck and femoral great trochanter BMD [40, 47]. In addition, Qianggu Capsule had a more significant effect on femoral neck and femoral trochanteric BMD compared withα-D3 capsule [42, 43, 45]. No severe ADRs were found and the common ADRs could be improved promptly without special treatment.
So far, there is only one systematic review reporting Qianggu Capsule in treating POP [54]. Compared with previously reported review, our study strictly followed the PRISMA statement and added more randomised controlled trials. Secondly, the control groups were limited to be no treatment, placebo or conventional therapy. As well, for many complementary and alternative treatments there were not enough information about their efficacy and safety. So the alternative interventions were not enrolled as controls. Thirdly, we also summarized and analyzed the objective quantized outcomes, including bone formation and resorption markers.
Recommendation on the Efficacy evaluation of Qianggu Capsule in the treatment of POP
In our study, definite conclusions could not be drawn in some subgroups because of the limited trials [38, 39, 41, 44, 46]. The meta-analysis was performed according to the homogeneity of the trials. Based on the current data, osteoporotic-fractures, quality of life and the related symptoms were not designed or evaluated in the included trials. BMD and metabolic markers were the most frequently reported outcomes. However, the results of Meta-analysis across trials were hampered by the high risk of bias, inconsistent result in some analysis, and small sample sizes (<400) on the basis of the GRADE approach. Eventually levels of quality of evidence were evaluated as low or very low. Thus, interpretation of these positive findings should be cautions.
On the other hand, the available meta-analysis did not confirm the efficacy for biochemical markers of bone turnover. The level of evidence was evaluated to be very low. One possible reason was the small sample sizes and short-term treatment. Meanwhile, some important bone turnover markers were not used for the diagnosis or evaluation in the primary studies. Accordingly, we suggest that serum procollagen type I amino-terminal propeptide (PINP) andβ-isomerised carboxy-terminal cross-linking telopeptide of type I collagen (CTX) be used as one of the important index, especially for the evaluation [55, 56].
Limitation of this systematic review and direction for further clinical research
There are a number of methodological weaknesses in the previous studies. The majority of the included trials did not provide inadequate reporting of random method and allocation concealment. Only 1 trial used placebo-controlled design in our review [44]. Blinding is necessary to avoid detection bias. Randomized clinical trials without placebo design were likely to generate false positive results, such as the add-on design features (A + B versus B) [57]. It is difficult to evaluate the Qianggu Capsule absolute efficacy without a true placebo. Two trials did not report information on drop-out and withdraws [40, 44]. None of the included trials reported a pretrial estimation of sample size. All the studies were not large-scale randomized clinical trials. Since all the trials were published in Chinese journals, we could not exclude the potential publication bias.
Greater attention to methodological quality continues to be needed. In the future, large-sample and high-quality randomised, placebo-controlled trials should be conducted to further confirm the efficacy of Qianggu Capsule in treating POP. Since POP is a chronic metabolic disease, the effect of long-term treatment is a great concern of patients.
Conclusions
Qianggu Capsule alone or Qianggu Capsule plus Caltrate D were beneficial for POP patients comparing to conventional interventions in improving BMD. Nevertheless, the evidence level was assessed to be low or very low according to GRADE approach. Therefore, the interpretation of that potential efficacy should be cautious, further research with strictly designed method is needed. Adverse outcomes of Qianggu Capsule mainly included constipation and dry mouth.
Abbreviations
- ADR:
-
Adverse drug reaction
- BMD:
-
Bone mineral density
- CBM:
-
Chinese Biomedical Literature Database
- CI:
-
Confidence intervals
- CNKI:
-
Chinese national knowledge infrastructure
- CTX:
-
β-isomerised carboxy-terminal cross-linking telopeptide of type I collagen
- MD:
-
Mean difference
- PINP:
-
Procollagen type I amino-terminal propeptide
- POP:
-
Primary osteoporosis
- RR:
-
Risk ratio
- SMD:
-
Standardized mean difference
- VIP:
-
Chinese Scientific Journals Database
References
Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–64.
Crandall CJ. Risk assessment tools for osteoporosis screening in postmenopausal women: a systematic review. Curr Osteoporos Rep. 2015;13:287–301.
Cooper C, Cole ZA, Holroyd CR, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22:1277–88.
Lin X, Xiong D, Peng YQ, Sheng ZF, Wu XY, Wu XP, Wu F, Yuan LQ, Liao EY. Epidemiology and management of osteoporosis in the People’s Republic of China: current perspectives. Clin Interv Aging. 2015;10:1017–33.
Xia WB, He SL, Xu L, Liu AM, Jiang Y, Li M, Wang O, Xing XP, Sun Y, Cummings SR. Rapidly increasing rates of hip fracture in Beijing. China J Bone Miner Res. 2012;27(1):125–9.
Xie Z, Burge R, Yang Y, Du F, Lu T, Huang Q, Ye W, Xu W. Posthospital discharge medical care costs and family burden associated with osteoporotic fracture patients in china from 2011 to 2013. J Osteoporos. 2015;2015:258089.
Bernabei R, Martone AM, Ortolani E, Landi F, Marzetti E. Screening, diagnosis and treatment of osteoporosis: a brief review. Clin Cases Miner Bone Metab. 2014;11(3):201–7.
Nazrun AS, Tzar MN, Mokhtar SA, Mohamed IN. A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. Ther Clin Risk Manag. 2014;10:937–48.
Modi A, Tang J, Sen S, Díez-Pérez A. Osteoporotic fracture rate among women with at least 1 year of adherence to osteoporosis treatment. Curr Med Res Opin. 2015;31(4):767–77.
Wu XP, Hou YL, Zhang H, Shan PF, Zhao Q, Cao XZ, Dai RC, Luo XH, Liao EY. Establishment of BMD reference databases for the diagnosis and evaluation of osteoporosis in central southern Chinese men. J Bone Miner Metab. 2008;26(6):586–94.
Burch J, Rice S, Yang H, Neilson A, Stirk L, Francis R, Holloway P, Selby P, Craig D. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess. 2014;18(11):1–180.
Songpatanasilp T, Mumtaz M, Chhabra H, Yu M, Sorsaburu S. Back pain in patients with severe osteoporosis on teriparatide or antiresorptives: a prospective observational study in a multiethnic population. Singapore Med J. 2014;55(9):493–501.
Ljunggren Ö, Barrett A, Stoykov I, Langdahl BL, Lems WF, Walsh JB, Fahrleitner-Pammer A, Rajzbaum G, Jakob F, Karras D, Marin F. Effective osteoporosis treatment with teriparatide is associated with enhanced quality of life in postmenopausal women with osteoporosis: the European Forsteo Observational Study. BMC Musculoskelet Disord. 2013;14:251.
Edwards BJ, Bunta AD, Lane J, Odvina C, Rao DS, Raisch DW, McKoy JM, Omar I, Belknap SM, Garg V, Hahr AJ, Samaras AT, Fisher MJ, West DP, Langman CB, Stern PH. Bisphosphonates and nonhealing femoral fractures: analysis of the FDA Adverse Event Reporting System (FAERS) and international safety efforts: a systematic review from the Research on Adverse Drug Events And Reports (RADAR) project. J Bone Joint Surg Am. 2013;95(4):297–307.
Netelenbos JC, Geusens PP, Ypma G, Buijs SJ. Adherence and profile of non-persistence in patients treated for osteoporosis—a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int. 2011;22(5):1537–46.
Hiligsmann M, Bours SP, Boonen A. A review of patient preferences for osteoporosis drug treatment. Curr Rheumatol Rep. 2015;17(9):533.
Tadrous M, Wong L, Mamdani MM, Juurlink DN, Krahn MD, Lévesque LE, Cadarette SM. Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: a network meta-analysis. Osteoporos Int. 2014;25(4):1225–35.
Fadda V, Maratea D, Trippoli S, Messori A. Gastrointestinal and renal side effects of bisphosphonates: differentiating between no proof of difference and proof of no difference. J Endocrinol Invest. 2015;38(2):189–92.
Reginster JY, Pelousse F, Bruyère O. Safety concerns with the long-term management of osteoporosis. Expert Opin Drug Saf. 2013;12(4):507–22.
Zhu HM, Qin L, Garnero P, Genant HK, Zhang G, Dai K, Yao X, Gu G, Hao Y, Li Z, Zhao Y, Li W, Yang J, Zhao X, Shi D, Fuerst T, Lu Y, Li H, Zhang X, Li C, Zhao J, Wu Q, Zhao SJ. The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis. Osteoporos Int. 2012;23(4):1317–27.
Wang ZQ, Li JL, Sun YL, Yao M, Gao J, Yang Z, Shi Q, Cui XJ, Wang YJ. Chinese herbal medicine for osteoporosis: a systematic review of randomized controlled trails. Evid Based Complement Alternat Med. 2013;2013:356260.
Huang Q, Shi J, Gao B, Zhang HY, Fan J, Li XJ, Fan JZ, Han YH, Zhang JK, Yang L, Luo ZJ, Liu J. Gastrodin: an ancient Chinese herbal medicine as a source for anti-osteoporosis agents via reducing reactive oxygen species. Bone. 2015;73:132–44.
Wang JN, Jiang JJ, Xie YM, Wei X, Li JP, Duan JL, Xiong X. Population pharmacokinetics of naringin in total flavonoids of Drynaria fortunei (Kunze) J. Sm. in Chinese women with primary osteoporosis. Chin J Integr Med. 2012;18(12):925–33.
Xie YM, Yuwen Y, Dong FH, Sun SC, Wang HM, Liu QS, Hua ZJ, Ma LX, Liao X, Xu GQ, Zhi YJ, Niu LF, Wu CS. Clinical practice guideline of traditional medicine for primary osteoporosis. Chin J Integr Med. 2011;17:52–63.
Jiannong W, Junjie J, Yanming X, Xu W, Jianpeng L, Jingli D, Xin X. Effect of naringenin in Qianggu capsule on population pharmacokinetics in Chinese women with primary osteoporosis. J Tradit Chin Med. 2015;35(2):141–53.
Wei X, Li JP, Xie YM. TCM commonly used treatment method and research progress of four Chinese medicine treatment for postmenopausal osteoporosis. Global Tradit Chin Med. 2011;4(6):481–5.
Kanis JA, Melton 3rd LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–41.
Liu ZH, Zhao YL, Ding GZ, Zhou Y. Epidemiology of primary osteoporosis in china. Osteoporos Int. 1997;7:S84–7.
Liu ZH, Yang DZ, Zhu HM, Wang HF, Zhang L. Diagnostic standard of primary osteoporosis in China. Chin J Osteoporosis. 1999;5:1–4.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Furlan AD, Pennick V, Bombardier C, van Tulder M, Cochrane Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine. 2009;34(18):1929–41.
Gross A, Miller J, D’Sylva J, Burnie SJ, Goldsmith CH, Graham N, Haines T, Brønfort G, Hoving JL. Manipulation or Mobilisation for Neck Pain. Cochrane Database Syst Rev. 2010;(1):CD004249.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams Jr JW, Zaza S, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490.
Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori V, Jaeschke R, Rind D, Dahm P, Meerpohl J, Vist G, Berliner E, Norris S, Falck-Ytter Y, Murad MH, Schünemann HJ, GRADE Working Group. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–6.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Gu M, Guo JN. Gusuibu treatment for primary osteoporosis. Chin Rehabil. 2004;19(5):297.
Zhao G, Xu ZL, Shao QX, Feng JL, Xue JP, Wang JG, Yang HX, Li R, Li YJ. Confarison of livial and kidney-invigorating traditional Chinese medicine in prevention and treatment of postmenopausal osteoporosis. Chin J Osteoporosis. 2004;10(3):337–9.
Xia WF, Chen LL. Comparison of traditional Chinese medicine (Qiang-gu capsule) and Risedronate sodium in management of postmenopausal osteoporosis. Chin J Osteoporosis. 2006;12(4):393–6.
Ji XR. The clinical observation of Qianggu capsule treatment for senile osteoporosis. Shanxi Med. 2006;35(4):341–2.
Shan S, Zhou G. The clinical effect of Qianggu capsule for primary osteoporosis. Northwest Pharm. 2006;21(4):177–8.
Wang J, Zhang WK, Wang ZH. 28 cases of Qianggu capsule for postmenopausal osteoporosis. Chin Med Hera. 2007;26(11):1325–7.
Li JH, Zhao GL. The clinical study of Qianggu capsule in management of postmenopausal osteoporosis. Appl J Integr Med. 2008;8(6):19–20.
Gao LX. Clinical observation of Qianggu capsule treatment for primary osteoporosis. Wuhan: Hubei Univ Chin Med; 2008. p. 1–43.
Xu H, Ren DH, Liang Z, Wang J. Clinical observation of Qianggu capsule plus Alendronate on postmenopausal osteoporosis. Zhejiang J Univ Chin Med. 2010;34(4):503–4.
Zeng N, Wang YY, Qiu HJ. The influence of total flavonoids of Gusuibu for bone pain and bone mineral density in senile osteoporosis patients. Shandong Tradit Chin Med. 2013;32(6):387–8.
Guo Y, Li Y, Xue L, Severino RP, Gao S, Niu J, Qin LP, Zhang D, Brömme D. Salvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J Ethnopharmacol. 2014;155(3):1401–16.
Liu R, Kang X, Xu L, Nian H, Yang X, Shi H, Wang X. Effect of the combined extracts of herba epimedii and fructus ligustri lucidi on sex hormone functional levels in osteoporosis rats. Evid Based Complement Alternat Med. 2015;2015:184802.
Liu RH, Kang X, Xu LP, Nian HL, Yang XW, Shi HT, Wang XJ. Effects of the combined extracts of Herba Epimedii and Fructus Ligustri Lucidi on bone mineral content and bone turnover in osteoporotic rats. BMC Complement Altern Med. 2015;15:112.
Wang L, Li Y, Guo Y, Ma R, Fu M, Niu J, Gao S, Zhang D. Herba epimedii: an ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Curr Pharm Des. 2016;22(3):328–49.
Kang SN, Lee JS, Park JH, Cho JH, Park JH, Cho KK, Lee OH, Kim IS. In vitro anti-osteoporosis properties of diverse Korean Drynariae rhizoma phenolic extracts. Nutrients. 2014;6(4):1737–51.
Huang Y, Liu X, Zhao L, Li F, Xiong Z. Kidney tissue targeted metabolic profiling of glucocorticoid-induced osteoporosis and the proposed therapeutic effects of Rhizoma Drynariae studied using UHPLC/MS/MS. Biomed Chromatogr. 2014;28(6):878–84.
Chen YH, Li ZH, Cui XL, Liu M, Liu TS, Xiao SJ. A systematic review of Qianggu capsule for treatment of primary osteoporosis. Chin J Osteoporosis. 2010;16(9):652–4. 65.
Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA. IOF-IFCC Bone Marker Standards Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420.
Dai Z, Wang R, Ang LW, Yuan JM, Koh WP. Bone turnover biomarkers and risk of osteoporotic hip fracture in an Asian population. Bone. 2016;83:171–7.
Ernst E, Lee MS. A trial design that generates only “positive” results. J Postgrad Med. 2008;54(3):214–6.
Acknowledgements
We thank Dr. YK Yin from Indiana University Purdue University Indianapolis for language improvement of the manuscript.
Funding
YMX was supported by the National Natural Science Foundation of China (no. 81373885) and Clinical Base Project of State Administration of Traditional Chinese Medicine (no. JDZX2015076). XW was supported by Science and Technology Program of Beijing Administration of Traditional Chinese Medicine (no. JJ2015-57).
Availability of data and materials
All data generated or analysed during this study are included in these published articles [38–47].
Authors’ contributions
XW coordinated the systematic review, assessed methodological quality, performed meta-analysis of the review, and also drafted the manuscript. ALX participated in literature search, data abstraction and quality assessment. HS participated in literature search, data abstraction. YMX lead the conceptual design of the review and manuscript, verified data and provided content expertise. All authors read and approved the final manuscript.
Competing interests
Each author certifies that they have no commercial associations that might pose a conflict of interest connection with the submitted article.
Consent for publication
All the authors agree to publish the manuscript.
Ethics approval and consent to participate
Because the study is systematic review, so this section is not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wei, X., Xu, A., Shen, H. et al. Qianggu capsule for the treatment of primary osteoporosis: evidence from a Chinese patent medicine. BMC Complement Altern Med 17, 108 (2017). https://doi.org/10.1186/s12906-017-1617-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-017-1617-3