Abstract
Purpose
The aim of this study was to estimate the effective dose 90% (ED90) of carbetocin to provide adequate uterine tone at Cesarean delivery (CD) for labour arrest.
Methods
We conducted a double-blind dose-finding study of carbetocin using a biased-coin up-and-down design in women undergoing CD for labour arrest under epidural anesthesia. Forty healthy term pregnant women who had received at least three hours of oxytocin infusion during labour were recruited for the study. Carbetocin was administered intravenously upon delivery of the anterior shoulder of the fetus. The first patient received 20 µg, and the dose for the subsequent patient was determined according to the response of the previous patient as per the biased-coin allocation scheme using increments or decrements of 20 µg (maximum 140 µg). Uterine tone was assessed by the obstetrician and rated as satisfactory or unsatisfactory throughout the intraoperative period. The primary outcome was satisfactory uterine tone with no need for additional uterotonic drugs intraoperatively. Secondary outcomes included use of additional uterotonic drugs postoperatively in the first 24 hr, estimated blood loss, and adverse effects.
Results
The ED90 of carbetocin to produce adequate uterine tone was estimated at 121 µg (95% confidence interval [CI]: 111 to 130; 99% CI: 108 to 133) using the truncated Dixon and Mood (DM) method. The isotonic estimator of ED90 was 140 µg; however, the observed response rate across all doses was < 90%. Also, the 95% CI of the DM estimator is likely to have lower than expected coverage, while the 99% CI may have about 90% coverage. Therefore, these results should be interpreted with caution. The overall median (range) estimated blood loss was 1,014 (104-2,436) mL. The overall incidence of hypotension and tachycardia were 45% and 57.5%, respectively. At a dose of 140 µg, the incidence of tachycardia and intraoperative arrhythmias was 76% and 14%, respectively.
Conclusion
The ED90 of carbetocin at CD for labour arrest, as determined in our study, should be interpreted with caution since it may be underestimated. This dose is higher than the currently recommended dose of 100 µg at elective CD and should not be used routinely given the uncertainty regarding its efficacy and the high incidence of arrhythmias at higher doses. This trial was registered at ClinicalTrials.gov, number: NCT01725243.
Résumé
Objectif
L’objectif de cette étude était d’estimer la dose efficace à 90 % (DE90) de la carbétocine pour atteindre un tonus utérin adapté lors d’un accouchement par césarienne (AC) pour arrêt de la progression du travail.
Méthode
Nous avons réalisé une étude de détermination de dose de carbétocine à double insu en nous fondant sur une méthodologie biaisée de pile ou face chez des femmes subissant un AC pour arrêt de progression du travail sous anesthésie péridurale. Quarante femmes enceintes en bonne santé et à terme ayant reçu une perfusion d’oxytocine pendant au moins trois heures pendant le travail obstétrical ont été recrutées pour cette étude. La carbétocine a été administrée par voie intraveineuse lors de la sortie de l’épaule antérieure du fœtus. La première patiente a reçu 20 µg, et la dose pour la patiente suivante a été déterminée en fonction de la réaction de la patiente précédente selon un schéma d’attribution biaisé par pile ou face en utilisant des augmentations ou des réductions de 20 µg (maximum 140 µg). Le tonus utérin a été évalué par l’obstétricien et jugé satisfaisant ou insatisfaisant tout au long de la période peropératoire. Le critère d’évaluation principal était un tonus utérin satisfaisant sans nécessiter d’agents utérotoniques supplémentaires pendant l’intervention. Les critères d’évaluation secondaires comprenaient l’utilisation postopératoire d’agents utérotoniques supplémentaires au cours des premières 24 heures, la perte de sang estimée et les effets secondaires néfastes.
Résultats
La DE90 de carbétocine afin d’obtenir un tonus utérin adapté a été estimée à 121 µg (intervalle de confiance [IC] 95 %: 111 à 130; IC 99 %: 108 à 133) à l’aide de la méthode Dixon et Mood (DM) tronquée. L’estimateur isotonique de la DE90 était 140 µg; toutefois, le taux de réponse observé était < 90 %, toutes doses confondues. En outre, il est possible que l’IC 95 % de l’estimateur DM ait une couverture plus basse que prévue, alors que l’IC 99 % pourrait avoir une couverture d’environ 90 %. Par conséquent, il convient de faire preuve de prudence dans l’interprétation de ces résultats. La perte sanguine globale médiane estimée (intervalle) était de 1014 (140-2436) mL. L’incidence globale d’hypotension et de tachycardie était de 45 % et 57,5 %, respectivement. À une dose de 140 µg, l’incidence de tachycardie et d’arythmies peropératoires était de 76 % et 14 %, respectivement.
Conclusion
La DE90 de carbétocine en cas d’AC suite à un arrêt de progression du travail obstétrical, telle que déterminée dans notre étude, doit être interprétée avec prudence étant donné qu’elle pourrait être sous-estimée. Cette dose est plus élevée que la dose actuellement recommandée de 100 µg pour un AC non urgent et ne devrait pas être systématiquement administrée étant donné les incertitudes quant à son efficacité et à l’incidence élevée d’arythmies à des doses plus élevées. Cette étude a été enregistrée au numéro ClinicalTrials.gov: NCT01725243.
Similar content being viewed by others
Hemorrhage stands as the leading cause of maternal death, responsible for approximately 120,000 (34%) of the total maternal deaths annually worldwide.1 The most recent Centre for Maternal and Child Enquiries report Saving Mothers Lives - Reviewing maternal deaths to make motherhood safer: 2006-2008 attributed nine deaths per 100,000 live births to hemorrhage in the UK.2 Postpartum hemorrhage (PPH) is currently the third leading cause of maternal death in Canada.3 The most prevalent root cause of PPH is uterine atony, responsible for over 70% of reported cases.4,5
Active management of the third stage of labour (AMTSL) is an important way of preventing PPH. A key component of AMTSL is the prophylactic administration of uterotonic drugs after delivery of the fetus, which has shown to reduce the incidence of PPH by 40%.6
Oxytocin remains the first-line uterotonic drug for both prevention and treatment of PPH; however, it has its own limitations. The half-life of oxytocin is only four to ten minutes; therefore, it requires an infusion for administration.7 Furthermore, it displays a high incidence of dose-dependent adverse effects. The search for an alternative ideal uterotonic drug continues, and carbetocin appears to be a promising substitute.
A synthetic oxytocin analogue, carbetocin [1-deamino-1-carba-2-tyrosine (0-methyl)-oxytocin] has been developed with favourable pharmacokinetics. By binding to the oxytocin receptor (OTR) with the same affinity, it mirrors the actions of oxytocin. Its advantages include a longer half-life of 40 min (four to ten times that of oxytocin) that facilitates an ease in dosing to just a bolus administration.8 A recent Cochrane review revealed that, when compared with oxytocin, use of carbetocin lessens the need for additional uterotonic drugs or uterine massage in both Cesarean and vaginal deliveries.9
The use of carbetocin as the first-line uterotonic drug at elective Cesarean delivery (CD) was introduced into the Society of Obstetricians and Gynaecologists of Canada (SOGC) 2009 Clinical Practice Guidelines.10 Instead of administering a continuous oxytocin infusion to prevent PPH, they recommend administering carbetocin 100 µg as an intravenous bolus over one minute.
As the current evidence for this recommended dose is weak,11 our group conducted a series of dose-finding studies of carbetocin in women undergoing elective CD.12-14 We initially conducted two randomized controlled trials using doses of 80-120 µg and 20-100 µg and found satisfactory uterine response in 87% and 94% of patients, respectively. Since successful treatment was evenly distributed across all study groups, we could not plot a dose-response curve or estimate the effective dose 90% (ED90).12,13 Finally, using a biased-coin up-down design, our group recently estimated the ED90 of carbetocin to be 14.8 µg (95% confidence interval [CI]: 13.7 to 15.8).14
Although we have determined the ED90 of carbetocin at elective CD, its ED90 at CD for labour arrest remains unknown. Similar to our findings for oxytocin,15 it is expected that the ED90 of carbetocin in women who have undergone labour augmentation with oxytocin will be higher as a result of OTR desensitization. Hence, the primary objective of this study was to establish the ED90 of carbetocin at CD for labour arrest.
Methods
After obtaining approval from the Mount Sinai Hospital Research Ethics Board (REB 12-0218-A) and Health Canada (159256), this study was conducted at Mount Sinai Hospital (Toronto, ON, Canada), and patients were enrolled over a five-month period (November 2012-March 2013). The patient volume at our hospital is about 7,000 deliveries per year, and the catchment area is mainly the Greater Toronto Area and the province of Ontario. It serves as both a primary and a referral centre. The study was undertaken as a double-blind, biased-coin up-down sequential dose-finding trial. Written informed consent was obtained from all patients enrolled in the study.
Women undergoing low transverse CD secondary to labour arrest under epidural anesthesia were approached for participation in the study. Patients with pre-existing labour epidurals were chosen in order to provide a homogeneous patient population and to avoid any confounders or bias in the results. Inclusion criteria were term (> 37 weeks) gestation, singleton pregnancies, American Society of Anesthesiologists physical status class I and II, and at least three hours of oxytocin infusion for labour augmentation. Exclusion criteria were known drug allergy to oxytocin, risk factors for PPH (including invasive placentation, uterine fibroids, chorioamnionitis, multiple gestation, macrosomia, polyhydramnios, and history of PPH or uterine atony), pre-eclampsia, previous classical uterine incision, bleeding diathesis, and CD for fetal distress. Standard definitions were used for identifying these conditions based on the diagnoses made by the obstetricians as per SOGC guidelines.16-18
Obstetric data were recorded, including cervical dilatation and station of descent at the time of the diagnosis of labour arrest. The maximum rate of oxytocin infusion, the total dose of oxytocin administered, and the duration of oxytocin infusion were noted. The baseline vitals recorded were a mean of three systolic blood pressure (BP) readings, which were taken in the labour room using an automated noninvasive BP (NiBP) device, and heart rate (HR). The patient was positioned supine with a left lateral tilt on the operating table. The pre-existing epidural was topped up using 2% lidocaine 15-25 mL with 1:200,000 epinephrine and fentanyl 50 µg to achieve a bilateral sensory block to the T4 dermatome. Lactated Ringer’s solution was given via an 18G intravenous cannula as a co-load of 10 mL·kg−1 during the epidural top up and then maintained at 150 mL·hr−1. Standard monitoring included 5-lead electrocardiography, NiBP, and pulse oximetry. Blood pressure and HR were recorded every minute until ten minutes after delivery and then every 2.5 min until the end of surgery. Systolic BP was maintained within 10% of baseline with boluses of phenylephrine 0.1 mg.
The allocation scheme for the dose of carbetocin was prepared by the statistician based on the biased-coin up-down design targeting ED90. The statistician then gave the allocation scheme to a research assistant not involved in the study who then concealed the instructions for the desired dose and vials of carbetocin in opaque envelopes. Once a patient “passed” or “failed”, the result was entered onto a spreadsheet (which performed the random 1/9 number generation), and the next dose was then determined and placed in a sealed envelope.
The dose of carbetocin for each patient was determined by the response of the previous patient to the drug according to the allocation scheme. The first patient received a 20-µg dose based on the results of a study by Khan et al. 14 in patients undergoing elective CD, which showed an ED90 of 14.8 µg (95% CI: 13.7 to 15.8). If the patient did not respond adequately to the first dose, the dose for the next patient was increased by 20 µg. If the patient responded to a given dose, the dose for the next patient was either reduced by 20 µg with a probability of 1/9 or remained unchanged. In the event that a patient responded to the 20-µg dose, if the allocation scheme had determined a reduction in the dose, the decrement would have been 10 µg only. The study dose levels chosen were 10, 20, 40, 60, 80, 100, 120 µg to a maximum of 140 µg. The upper limit dose of 140 µg was chosen by analogy based on results of previous studies on oxytocin showing the ED90 during CD in labouring women (2.99 IU) to be about 8.5 times higher than in non-labouring women (0.35 IU).15,19 The ED90 of carbetocin in non-labouring women was recently found to be 14.8 µg by Khan et al.,14 and if we presume the same relationship with carbetocin as with oxytocin, then an amount 8.5 times higher would be 126.4 µg. We hypothesized that the ED90 of carbetocin for labouring women would fall in the dose range of 15-140 µg.
The study dose of carbetocin was diluted to 10 mL just before the start of CD by an anesthesiologist not involved in the study, and the investigator injected the drug intravenously over one minute immediately after the delivery of the anterior shoulder of the fetus. Staff obstetricians were given the following instructions prior to the start of the study: (i) carbetocin to be injected by the anesthesiologist over one minute immediately after delivery of the anterior shoulder; (ii) obstetricians to perform assisted spontaneous delivery of the placenta by applying cord traction rather than by active manual extraction of the placenta; (iii) no uterine massage to be performed within three minutes of carbetocin administration; and (iv) obstetricians to grade the uterine tone (UT) as satisfactory (firm) or unsatisfactory (boggy) by depressing the uterus with one finger. The tone would be considered satisfactory if the uterus could not be depressed with a finger or if it could be depressed only slightly but sprung back. On the other hand, a uterus that was soft and easily depressed would be considered to have unsatisfactory tone. The obstetricians performed UT assessments at two, three, four, five, and ten minutes after carbetocin administration and at any time during the operation as deemed necessary. If the UT was deemed inadequate at any point after two minutes following the completion of carbetocin administration, the obstetrician could request administration of additional uterotonic, in which case, the response to carbetocin treatment was considered a failure. In case of unsatisfactory UT and upon the obstetrician’s request, a bolus of oxytocin 3 IU was administered, followed by an infusion of 40 mU·min−1 (20 IU in 1,000 mL Ringer’s lactate solution at a rate of 120 mL·hr−1). If the UT continued to be poor, ergometrine and/or carboprost were given as appropriate. If no additional uterotonic drugs were required intraoperatively, the response to carbetocin treatment was considered a success.
The primary outcome was satisfactory UT with no need for additional uterotonic drugs intraoperatively. Secondary outcomes included: a) UT every minute for five minutes and at ten minutes following the completion of the injection of carbetocin, and postoperatively within two hours of delivery; b) need for delayed additional uterotonics within 24 hr after delivery outside the operating room; c) estimated blood loss; and d) the following adverse effects: intraoperative hypotension (a 20% decrease in systolic BP from baseline despite the use of prophylactic vasopressors), hypertension (a 20% increase in systolic BP from baseline), bradycardia (30% decrease in HR from baseline), tachycardia (30% increase in HR from baseline), nausea (as reported by the patients) and vomiting, chest pain, shortness of breath, headache, flushing and arrhythmias. Blood loss was estimated using the hematocrit variation method by measuring the difference in hematocrit values assessed before and at 24 hr after delivery according to the following formula: estimated blood volume (preoperative hematocrit - postoperative hematocrit)/ preoperative hematocrit, where estimated blood volume in mL is measured as the patient’s weight in kilograms × 85.Footnote 1
Sample size and statistical analysis
In this study, a biased-coin up-down design was used to target ED90. The dose of carbetocin for each patient was determined by the previous patient’s response. For example, if the previous patient responded to the administered dose, the current patient was randomized to the next lower dose with the probability of 1/9 [= (1-90%)/90%] and to the same dose with the probability of 8/9 (= 1-1/9).
For a biased-coin up-down study, the distribution of data is unknown and non-independent. Simulation studies suggest that enrolling at least 20-40 patients will provide stable estimates of the target dose; therefore we enrolled 40 patients in this study.20
Descriptive statistical methods were used to summarize the study population. The ED90 and 95% CI were estimated using two non-parametric methods, the truncated Dixon and Mood method (simple average of Brownlee, Hodges and Rosenblatt)21 and the isotonic regression method with the pooled-adjacent-violators algorithm (PAVA) approach with linear calibration.22 The latter was used only to confirm our findings and supplement our results. Patient characteristics and secondary outcomes were presented as median (range) or n (%). The statistical analyses were performed using SAS® 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
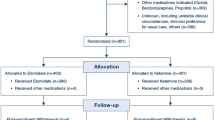
Out of the 49 women approached to participate, 40 completed the study and their data were analyzed (Fig. 1). Their mean (SD) age and body mass index were 32.9 (5.7) yr and 30.3 (4.1) kg·m−2, respectively. The mean (SD) gestational age was 39.7 (1.3) weeks. Thirty-three (82.5%) of the CDs were indicated for arrest of dilatation, whereas the remaining 7 (17.5%) were due to arrest of descent at full dilatation. All patients received oxytocin for either induction or augmentation of labour. The mean (SD) total amount of oxytocin received was 4,827 (2,942) mU administered for an average (SD) of 11.9 (6.9) hr during labour. The patient characteristics and obstetric data across all dose levels are shown in Table 1. Fourteen obstetricians were involved in the study with a median (range) number of cases of 3 (1-5).
The sequence of administered carbetocin doses and the response (success or failure) for each woman at their assigned dose level is displayed in Fig. 2. The observed response rate is shown in Table 2. Overall, 29 (72.5%) women had adequate UT intraoperatively, and 11 (27.5%) required additional uterotonics. Three women not only required a rescue with oxytocin, but they also required a third- and fourth-line uterotonic agent in the form of ergometrine 250 µg iv and an intramyometrial injection of carboprost 250 µg. Only one woman who initially responded with satisfactory UT in the operating room required additional uterotonic in the form of an oxytocin infusion postoperatively within two hours following carbetocin administration. None of the patients required any additional uterotonics in the subsequent 24 hr after delivery. Uterine massage was required in nine women (23%). Exteriorization of the uterus was at the discretion of the obstetrician and was performed in 72% of cases.
The ED90 of carbetocin to produce adequate UT was estimated at 121 µg (95% CI: 111 to 130) using the truncated Dixon and Mood method (simple average of Brownlee, Hodges, and Rosenblatt). The 99% CI was 108 to 133 µg. The estimated ED90 using isotonic regression with the PAVA approach was 140 µg; however, since the observed response rate across all doses was < 90%, the Dixon and Mood estimate of ED90 may be underestimated. Furthermore, the 95% CI of the Dixon and Mood estimate may have lower coverage than expected; therefore, to be more conservative, we have also provided the 99% CI which may have coverage of 90% (Appendix; available as Electronic Supplementary Material). Consequently, the results should be interpreted with caution.
The overall median (range) estimated blood loss was 1,014 (104-2,436) mL. The incidence of hypotension and hypertension was 45% and 25%, respectively, while nausea occurred in 37.5% of cases. The median (range) dose of phenylephrine used in the post-delivery period was 0.1 (0-1.1) mg, and the overall incidence of tachycardia and arrhythmias was 57.5% and 7.5%, respectively (Table 3). Among those who had received the 140 µg dose, 16 women (76.2%) developed tachycardia and three (14.3%) developed intraoperative arrhythmias. One of these patients suffered profound bradycardia (< 35 beats·min−1) during exteriorization of the uterus, for which she was given atropine 0.6 mg iv. Minutes later, the same patient developed sustained monomorphic ventricular tachycardia at a rate of 220 beats·min−1, followed by inferolateral ischemic ST changes on the electrocardiogram (ECG), associated chest pain, and a raised plasma troponin T level of over 300 ng·L−1. The episode resolved soon after pharmacotherapy, and her clinical symptoms of dyspnea and chest pain improved. A transthoracic echocardiogram was performed but revealed no abnormalities, and the computed tomography coronary angiogram showed small-calibre coronaries but no evidence of stenosis, dissection, or thrombosis. The cardiovascular episode was determined to be an exaggerated response to atropine in the form of ventricular tachycardia leading to rate-related ischemia. Following this case (study number 23), we collected pre- and 12-hr postoperative plasma troponin T levels and 12-lead ECG readings for the remaining 17 women. In these women, we observed no ECG changes or any significant rise in mean (SD) troponin T levels postoperatively [preoperative levels = 6.5 (3.4) ng·L−1; postoperative levels = 8.5 (3.1) ng·L−1]. One of the other two women who received the 140-µg dose had a self-resolving short run of bigeminal premature ventricular contractions, and the other had a self-resolving sinus arrhythmia intraoperatively. Both women suffered no clinical or hemodynamic sequelae from these recorded arrhythmias.
The median (range) blood loss in women with satisfactory UT was 19% lower than in women with unsatisfactory UT [961 (104-2,426) mL vs 1,187 (555-2,436) mL, respectively]. The median (range) blood loss in women requiring additional uterotonics was 34% more than in women who did not require additional uterotonics [1,271 (555-2,436) mL vs 951 (104-2,426) mL, respectively], although no statistically significant differences were observed.
There were no specific patient characteristics that could explain the last three consecutive patient failures at the clinical dose level of 140 µg. However, statistically we could apply a sensitivity analysis to calculate how significantly the last three consecutive failures would have affected our estimation. If we consider a scenario whereby the last two patients could be assigned to dose levels of 160 µg and 180 µg, respectively, the estimated ED90 would be 122.2 µg (95% CI: 111.9 to 132.4) using the same method, which evidently is very close to our original estimator of 120.5 µg.
Discussion
Our results indicate that, for women with oxytocin-augmented labour requiring CD for labour arrest in both first and second stage, the ED90 for carbetocin (121 µg; 95% CI: 111 to 130) is higher than the 100-µg dose recommended by SOGC for elective CD.10 Furthermore, this dose is at least eight times higher than the ED90 for carbetocin required in non-labouring women at full term undergoing elective CD (14.8 µg; 95% CI: 13.7 to 15.8).14
Despite the calculated ED90 at 121 µg, the observed response rate was < 90% across the studied dose range (20-140 µg); hence, the ED90 in our study may be underestimated. Nevertheless, considering the high incidence of complications at 140 µg, further studies should be cautiously planned after weighing the risks and benefits with the use of higher doses.
It is not surprising that the carbetocin dose requirement at CD for labour arrest is several times higher than at elective CD. Our own group has shown the same trend for oxytocin requirements. The ED90 of oxytocin in labouring women (2.99 IU; 95% CI: 2.32 to 3.62) was found to be 8.5 times higher than in non-labouring women (0.35 IU; 95% CI: 0.18 to 0.52) at term.15,19
This similar behaviour exhibited by the two uterotonic analogues in labouring and non-labouring women can be explained by the OTR desensitization phenomenon that has been extensively reported in the literature in in vitro studies.23,24 Just before the onset of labour, the concentration of OTRs and the sensitivity of the uterus to the effects of oxytocin increase significantly while preparing for parturition.25,26 As a result, low doses of oxytocin and carbetocin are typically effective for non-labouring women undergoing elective CD. On the other hand, in the subset of labouring women who fail to progress in labour, there is a decrease in OTR messenger RNA expression, density, and OTR binding sites, which results in the desensitization phenomenon.23-26
Our group has demonstrated the desensitization phenomenon in several in vitro experiments on isolated strips of both rat and human myometrium.27,28 A study in pregnant rat myometrium demonstrated that pre-exposure to oxytocin leads to a concentration-dependent attenuation of oxytocin-induced contractions as early as one hour after pre-exposure to oxytocin.27 Similarly, we demonstrated that oxytocin pretreatment attenuates oxytocin-induced contractility in human myometrium in both a concentration- and time-dependent manner.28 Furthermore, we also demonstrated that the human myometrial response to carbetocin is blunted by in vitro oxytocin pretreatment in a similar fashion to that of oxytocin.29 Our current study supports the idea that OTR signal transmission is attenuated in labouring women at the time of CD, and that this desensitization phenomenon also applies to the oxytocin analogue carbetocin.
Previous studies have shown that carbetocin 100 µg iv produces adverse effects comparable with those of oxytocin 5 IU iv, such as hypotension, tachycardia, nausea and vomiting, flushing, shortness of breath, and abdominal and chest pain.30,31 In our study, the overall incidence of hypotension with doses 20-140 µg was 45%, which is similar to the 37.5% incidence observed by Khan et al. 14 when using much lower doses of carbetocin (10-20 µg). These results are also in keeping with two other studies using a wide range of carbetocin doses.12,13 This may suggest that the speed of injection of the carbetocin bolus may be a more important determinant of the incidence of hypotension than the actual dose level. Nevertheless, these studies were not powered to look at differences in hypotension.
The higher incidence of tachycardia (76%) and other arrhythmias (14%) found at the 140-µg dose level is concerning and warrants careful attention to doses above 120 µg, especially when administered as boluses. Nevertheless, the one patient with profound intraoperative bradycardia had a medical history of vasovagal syncopal attacks, potentially exacerbating a vagal response to exteriorization of the uterus and increasing sensitivity to atropine. Having reported this case to Ferring Pharmaceuticals, we consider it to be a novel occurrence of profound bradycardia associated with the use of carbetocin. This case seeks to raise the awareness of the need for vigilance during CD with drugs causing potential coronary vasospasm, including uterotonic drugs as well as vagolytic drugs, e.g., atropine, even in patients without structural heart disease.
The overall incidence of post-delivery nausea and vomiting in our study was 37.5% and 10%, respectively, marginally higher than in the study conducted on carbetocin in elective CD by Khan et al. (27.5% and 7.5%, respectively).14 Nevertheless, this was lower than the incidence found with oxytocin at CD in labouring patients (60% and 40%, respectively).15 All these results must be interpreted with caution as these studies were not powered to look at side effects. Further studies are also required to clarify whether equipotent doses of both oxytocin and carbetocin are associated with different incidences of nausea and vomiting.
We acknowledge that there are some limitations to our study design, including the small sample size, which is attributed to the biased-coin up-down design. We chose this method because it is robust and efficient in providing the ED90 while subjecting fewer patients to ineffective doses of the study drug when compared with the classical randomized parallel group design.
The other limitation of our study is the subjective nature of the UT assessment determining the need for additional uterotonic agents. Nevertheless, this method has been previously used in the literature and reflects real clinical practice of the obstetricians to guide the use of uterotonic drugs. Furthermore, to date, an alternative validated objective method for the assessment of UT is lacking. In our institution, it is not practical or possible to have two staff obstetricians in every case; consequently, we did not attempt to establish the inter-rater reliability of the assessment of UT. Cesarean deliveries are typically performed by the staff obstetrician assisted by a fellow; hence, the staff obstetrician is the primary decision-maker. We had undertaken measures to ensure standardization of the process; for instance, prior to the start of CD, the obstetricians were given a set of instructions indicating how to assess the UT. Furthermore, over the last ten years, the obstetricians at our hospital were involved in several previous studies (total of 358 patients) that required UT assessment.12-15,19,32 They were familiar with the method, and hence, we expected consistency in their assessment.
The assessment of blood loss by the hematocrit equation is subject to criticism; however, this is the best method to date and is in keeping with the existing obstetric practice. Based on our data, satisfactory UT correlated with decreased blood loss. Despite lacking a statistically significant association, these data may serve to validate the assertion that, although there is subjectivity in the assessment of UT, the obstetricians were providing very useful information in predicting overall blood loss in their assessment.
The obstetricians were instructed not to carry out uterine massage within the first three minutes of carbetocin administration; this is because there could be differing thresholds for using uterine massage, which could represent a confounder in this study. Had early uterine massage been applied immediately after delivery in all cases in our study, it is possible that it may have led to a reduction in the ED90 of carbetocin. This may be another important explanation why the ED90 of carbetocin was higher than expected.33
Another limitation of our study is that uterine exteriorization, which is an important determinant of UT, was not standardized in the study and was left at the discretion of the obstetrician. This could have some effects on the ED90 of carbetocin and other results, such as nausea, vomiting, and visceral pain. In this study, the rate of uterine exteriorization was found to be high (72%) and comparable with the other studies,12-14 as the same group of obstetricians was involved.
In summary, our results suggest that the ED90 of carbetocin at CD for labour arrest in healthy women is higher than the recommended dose of 100 µg. To confirm our findings, we suggest performing future randomized controlled trials using predetermined doses above 100 µg in a larger patient population. Until such studies are completed, routine use of higher doses of carbetocin is not recommended due to the high incidence of arrhythmias at a dose of 140 µg in this study. Patients receiving higher doses should be carefully monitored.
It is possible that oxytocin analogues could fail in cases of labour arrest. Further studies looking into the optimum mode of delivering carbetocin, e.g., the speed of injection, may lead to a reduction in hemodynamic adverse effects as previously shown with oxytocin.
Notes
Shook PR, Schultz JR, Reynolds JD, Spahn TE, DeBalli P. Estimating blood loss for cesarean section. How accurate are we? Anesthesiology 2003; 98 (Supp 1): SOAP A2 (abstract).
References
World Health Organization, Unicef, UNFPA, The World Bank. Trends in Maternal Mortality 1990 to 2008: Estimates developed by WHO, Unicef, UNFPA and The World Bank. Geneva: World Health Organization 2010. Available from URL: http://www.who.int/reproductivehealth/publications/monitoring/9789241500265/en/ (accessed February 2015).
Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mother’s Lives: reviewing maternal deaths to make motherhood safer: 2006-2008.The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011; 118(Suppl. 1): 1-203.
Public Health Agency of Canada. Maternal Mortality in Canada. Available from URL: http://www.phac-aspc.gc.ca/rhs-ssg/ (accessed February 2015).
Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg 2010; 110: 1368-73.
Lutomski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum hemorrhage in Ireland: an 11-year population based cohort study. BJOG 2012; 119: 306-14.
Dyer RA, van Dyk D, Dresner A. The use of uterotonic drugs during caesarean section. Int J Obstet Anesth 2010; 19: 313-9.
Ryden G, Sjoholm I. Half-life of oxytocin in blood of pregnant and non-pregnant women. Acta Endocrinol (Copenh) 1969; 61: 425-31.
Barth T, Krejci I, Kupkova B, Jost K. Pharmacology of cyclic analogues of deamino-oxytocin not containing a disulphide bond (carba analogues). Eur J Pharmacol 1973; 24: 183-8.
Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012; 4: CD005457.
Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labor: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2009; 31: 980-93.
Peters NC, Duvekot JJ. Carbetocin for the prevention of postpartum hemorrhage: a systematic review. Obstet Gynecol Surv 2009; 64: 129-35.
Cordovani D, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose. Can J Anesth 2012; 59: 751-7.
Anandakrishnan S, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose - part 2. Can J Anesth 2013; 60: 1054-60.
Khan M, Balki M, Ahmed I, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a sequential allocation trial to determine the minimum effective dose. Can J Anesth 2014; 61: 242-8.
Balki M, Ronayne M, Davies S, et al. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstet Gynecol 2006; 107: 45-50.
Allen VM, Yudin MH, Bouchard M, et al. Management of group B streptoccal bacteruria in pregnancy. J Obstet Gynaecol Can 2012; 34: 482-6.
Liston R, Sawchuck D, Young D; Society of Obstetrics and Gynaecologists of Canada; British Columbia Perinatal Health Program. Fetal health surveillance: antepartum and intrapartum consensus guideline. J Obstet Gynaecol Can 2007; 29 (9 Suppl 4): S3-56.
Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can 2014; 36: 416-38.
Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol 2004; 104(5 Pt 1): 1005-10.
Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 2007; 107: 144-52.
Brownlee KA, Hodges JL Jr, Rosenblatt M. The up-and-down method with small samples. J Am Stat Assoc 1953; 48: 262-77.
Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics 2002; 58: 171-7.
Robinson C, Schumann R, Zhang P, Young RC. Oxytocin induced desensitization of the oxytocin receptor. Am J Obstet Gynecol 2003; 188: 497-502.
Phaneuf S, Rodriguez Linares B, TambyRaja RL, MacKenzie IZ, Lopez Bernal A. Loss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labour. J Reprod Fertil 2000; 120: 91-7.
Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of human oxytocin receptor. Nature 1992; 356: 526-9.
Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 1984; 150: 734-41.
Magalhaes JK, Carvalho JC, Parkes RK, Kingdom J, Li Y, Balki M. Oxytocin pretreatment decreases oxytocin-induced myometrial contractions in pregnant rats in a concentration-dependent but not time-dependent manner. Reprod Sci 2009; 16: 501-8.
Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology 2013; 119: 552-61.
Balki M, Cole N, Erik-Soussi M, Kingdom J, Carvalho JC. Carbetocin vs oxytocin: in-vitro contractions in oxytocin pre-treated myometrium. Can J Anesth 2012; 59(Suppl): Abstract (1344730).
Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlemback D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG 2011; 118: 1349-56.
Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology 2013; 119: 541-51.
Balki M, Dhumne S, Kasodekar S, Kingdom J, Windrim R, Carvalho JC. Oxytocin-ergometrine co-administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG 2008; 115: 579-84.
Soltani H. Uterine Massage for Preventing Postpartum Haemorrhage: RHL Commentary (last revised: 1 April 2010). The WHO Reproductive Health Library; Geneva: World Health Organization. Available from URL: http://apps.who.int/rhl/pregnancy_childbirth/childbirth/3rd_stage/Cd006431_soltanih_com/en/ (accessed February 2015).
Acknowledgements
The authors sincerely thank Kristi Downey MSc, Perinatal Research Coordinator, for her invaluable collaboration in all stages of this research project, including study design, Research Ethics Board application, data collection, and interpretation and elaboration of the manuscript.
Declaration of interests
Dr. Dan Farine is a consultant for Ferring Pharmaceutics and a member of its Medical Board.
Funding
This study was funded by Merit Awards, University of Toronto, and internally by the Department of Anesthesia and Pain Management, Mount Sinai Hospital, University of Toronto.
Disclosures
This paper was presented in the Best Paper Competition at the 45th Annual Meeting of the Society for Obstetric Anesthesia and Perinatology, Puerto Rico, April 24-28, 2013. It was also published as an abstract (Can J Anesth 2013: 60; S1-S121, Abstract 1653630) for the Annual Meeting of the Canadian Anesthesiologists’ Society, Calgary, Alberta, June 21-24, 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Nhathien Nguyen-Lu, Jose Carlos Almeida Carvalho, Mrinalini Balki, Dan Farine, Gareth Seaward, and Xiang Y. Ye participated in the research design. Nhathien Nguyen-Lu, Jose Carlos Almeida Carvalho, and Mrinalini Balki participated in data collection and manuscript writing. Dan Farine, Gareth Seaward, and Xiang Y. Ye reviewed the manuscript. Nhathien Nguyen-Lu, Jose Carlos Almeida Carvalho, Mrinalini Balki, and Xiang Y. Ye participated in data interpretation. Xiang Y. Ye performed the statistical analyses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen-Lu, N., Carvalho, J.C.A., Farine, D. et al. Carbetocin at Cesarean delivery for labour arrest: a sequential allocation trial to determine the effective dose. Can J Anesth/J Can Anesth 62, 866–874 (2015). https://doi.org/10.1007/s12630-015-0375-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0375-2