Abstract
Purpose
The purpose of this study was to determine the intravenous dose of carbetocin required to produce effective uterine contraction in 90% of females (ED90) undergoing elective Cesarean delivery (CD) under spinal anesthesia.
Methods
We conducted a double-blind dose-finding study of carbetocin. Forty females undergoing elective CD received carbetocin intravenously upon delivery of the fetus. The dose of carbetocin for each patient was determined according to a biased-coin up-and-down sequential allocation scheme designed to cluster doses close to ED90. The initial dose was 10 μg, with increments/decrements of 5 μg. The anesthesiologist, obstetrician, and patient were blinded to the dose. The obstetrician assessed the uterine tone at one-minute intervals for five minutes after carbetocin administration. In case of unsatisfactory tone, additional uterotonics were administered. The primary outcome was requirement for additional intraoperative uterotonics. Secondary outcomes were postoperative requirement for additional uterotonics within 24 hr of delivery, estimated blood loss and side effects.
Results
The ED90 of carbetocin was 14.8 μg (95% confidence interval 13.7 to 15.8). Thirty-seven patients (92.5%) had adequate uterine tone with no requirement of additional intraoperative uterotonics. Two patients (5%) required postoperative uterotonics within 24 hr. The overall mean (SD) estimated blood loss was 786 (403) mL and the overall incidence of hypotension (decrease in systolic blood pressure ≥ 20% baseline) was 37.5%.
Conclusion
Based on our study, the ED90 of carbetocin at elective CD is less than one-fifth the currently recommended dose of 100 μg. This study was registered at clinicaltrials.gov (NCT-01651130).
Résumé
Objectif
L’objectif de cette étude était de déterminer la dose intraveineuse de carbétocine nécessaire à produire une contraction utérine efficace chez 90 % des femmes (DE90) subissant un accouchement par césarienne programmé sous rachianesthésie.
Méthode
Nous avons réalisé une étude à double insu de détermination de dose de carbétocine. Quarante femmes subissant un accouchement par césarienne programmé ont reçu de la carbétocine par voie intraveineuse lors de l’accouchement du fœtus. Pour chaque patiente, la dose de carbétocine a été déterminée en fonction d’un schéma d’attribution séquentielle de doses croissantes ou décroissantes avec tirage biaisé conçu de façon à regrouper des doses près de la DE90. La dose initiale était de 10 μg, avec des augmentations ou réductions de 5 μg. L’anesthésiologiste, l’obstétricien et la patiente ne connaissaient pas la dose administrée. L’obstétricien a évalué le tonus utérin à des intervalles d’une minute pendant cinq minutes après l’administration de carbétocine. En cas de tonus insatisfaisant, des agents utérotoniques supplémentaires ont été administrés. Le critère d’évaluation principal était le besoin d’agents utérotoniques peropératoires supplémentaires. Les critères d’évaluation secondaires étaient le besoin postopératoire d’agents utérotoniques supplémentaires au cours des 24 h suivant l’accouchement et la perte de sang estimée; les effets secondaires ont été évalués.
Résultats
La DE90 de la carbétocine était de 14,8 μg (intervalle de confiance 95 % 13,7 à 15,8). Le tonus utérin de 37 patientes (92,5 %) était adéquat sans nécessiter d’agents utérotoniques peropératoires supplémentaires. Deux patientes (5 %) ont nécessité des agents utérotoniques postopératoires au cours des premières 24 h. La perte de sang estimée moyenne globale (ET) était de 785,6 (402,8) mL et l’incidence globale d’hypotension (réduction de la pression artérielle systolique ≥ 20 % de la valeur de base) était de 37,5 %.
Conclusion
Selon notre étude, la DE90 de carbétocine lors d’un accouchement par césarienne programmé est inférieure à un cinquième de la dose actuellement recommandée de 100 μg. Cette étude est enregistrée sous clinicaltrials.gov (NCT-01651130).
Similar content being viewed by others
Postpartum hemorrhage (PPH) is one of the leading causes of maternal morbidity and mortality worldwide, and in most cases, it is caused by uterine atony. The incidence of PPH in North America may be increasing.1,2 In a recent Canadian retrospective cohort study, the incidence of PPH increased from 4.1% in 1991 to 5.1% in 2004, and the rate of obstetric hysterectomies secondary to PPH increased from 24 to 41.7 per 100,000 deliveries in 1991 and 2004, respectively. The increase in incidence and severity of PPH has been attributed mainly to a higher frequency of atonic PPH, 29.4-39.5 per 1,000 deliveries during the same period.1 Similarly, using the National Inpatient Sample, a study from the United States reported an increase in the incidence of PPH from 2.3% in 1994 to 2.9% in 2006. This was also related to an increase in the rate of uterine atony from 1.6% in 1994 to 2.4% in 2006.2
It has long been recognized that the active management of the third stage of labour reduces the incidence and severity of PPH. Oxytocin is the most commonly used uterotonic and is recommended by the World Health Organization as the uterotonic of choice for the prevention of PPH.3 The administration of oxytocin immediately after the delivery of the fetus can reduce the incidence of PPH by up to 40%.4 The main limitation of oxytocin is its short half-life of four to ten minutes,5,6 which necessitates its use as an infusion. Furthermore, oxytocin has been associated with dose-dependent side effects such as hypotension, tachycardia, myocardial ischemia, arrhythmias, nausea, vomiting, headache, and flushing.7-9
Carbetocin [1-deamino-1-carba-2-tyrosine (0-methyl)-oxytocin] is a synthetic analogue of oxytocin that shares the same mechanism of action with oxytocin, thereby stimulating oxytocin receptors in the uterus.10 The main advantage of carbetocin is its longer half-life of approximately 40 min,11 which leads to a duration of action of about four to ten times that of oxytocin. Several trials8,12,13 and a recent review14 have compared the efficacy of carbetocin and oxytocin for the prevention of PPH following Cesarean deliveries (CD). The most consistent finding in such studies was the reduced requirement of additional uterotonics in patients who were administered carbetocin.
In 2009, the Society of Obstetricians and Gynecologists of Canada (SOGC) revisited their guidelines for the management of PPH and suggested carbetocin in the dose of 100 μg, administered intravenously over one minute, as the preferred uterotonic to prevent PPH in elective CD.15 Nevertheless, the evidence base for the use of carbetocin at a relatively high dose of 100 μg is unclear. Two previous dose-finding studies of carbetocin at elective CD conducted by our own group (with doses varying from 20-120 μg) have shown similar efficacy across all doses, with an incidence of hypotension in the range of 42.5-55%.16,17 It was suggested that the dose of carbetocin effective in 90% of females (ED90) at elective CD could be lower than 20 μg and hence should be sought in an attempt to minimize side effects, primarily hypotension.
The purpose of our study was to determine the ED90 of carbetocin at elective CD. We hypothesized, based on our previous dose-finding studies, that the ED90 would be less than 20 μg.
Methods
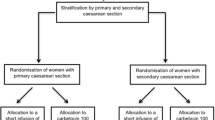
This prospective double-blind trial was performed from June 2012 to August 2012 following approval by the Research Ethics Board at Mount Sinai Hospital in Toronto, Canada (REB 12-0082-A, approval date May 23, 2012). Written informed consent was obtained from all females enrolled in the study. Inclusion criteria were healthy term pregnant females with singleton pregnancy scheduled for an elective CD under spinal anesthesia. Exclusion criteria were females with American Society of Anesthesiologists physical status III and above, requirement of general anesthesia, administration of nitroglycerine prior to delivery for uterine relaxation, and conditions predisposing to PPH, such as multiple gestation, abnormal placentation, uterine fibroids, chorioamnionitis, macrosomia, preeclampsia, uterine fibroids, history of PPH, or bleeding diathesis.
Baseline vital signs, including blood pressure (BP) and heart rate (HR), were recorded in the admitting unit using an automated noninvasive BP device, and a mean of three readings, obtained two minutes apart, was calculated. An 18G peripheral intravenous line was established through which lactated Ringer’s solution 10 mL·kg−1 was administered as co-load during spinal anesthesia administration. After skin disinfection and local infiltration, a 27G Whitacre needle was used to perform the spinal puncture at the L2-3 or L3-4 interspace with the patient in the sitting position. A dose of 0.75% hyperbaric bupivacaine 12-13.5 mg with fentanyl 10 μg and morphine 100 μg was injected intrathecally, following which the patient was immediately placed supine with left lateral displacement of the uterus using a wedge under the right buttock. Routine monitoring included electrocardiography, pulse oximetry, and noninvasive BP assessment. Heart rate and BP were recorded every minute until five minutes after delivery and then every 2.5 min until the completion of surgery. Phenylephrine boluses (100 μg iv) were administered as required to maintain BP at the patient’s baseline values.
Prior to the start of the CD, the obstetricians were requested to read a card with a set of instructions. The instructions were as follows: a) carbetocin to be injected (by the anesthesiologist) over one minute immediately after delivery of fetal head; b) cord traction to be applied for placental delivery without manual extraction; c) uterus to be depressed with one finger to assess the tone as firm (adequate) or boggy (inadequate) after one minute of carbetocin administration, for every minute until five minutes, and thereafter at the discretion of the obstetrician.
The attending anesthesiologist administered carbetocin intravenously over one minute immediately after the delivery of the fetal head. The anesthesiologist, obstetrician, and patient were blinded to the dose of carbetocin. Just before the CD, a research assistant not involved in the study prepared the carbetocin solution by diluting the required dose to 10 mL using normal saline.
The dose of carbetocin for each patient was determined by the previous patient’s response to the drug according to a biased-coin up-and-down sequential allocation scheme designed to cluster doses close to ED90.18,19 If a patient did not respond adequately to the carbetocin bolus, the initial dose for the next patient was increased by 5 μg. If a patient responded adequately, the dose for the next patient was decreased by 5 μg with a probability of 1/9; otherwise the dose remained unchanged. The biased-coin allocation after each successful response was implemented using a computer-generated list of random responses prepared by the statistician. One exception to this was the first patient who received a dose of 10 μg. The starting dose of 10 μg was based on the results of the study by Anandakrishnan et al.17 which showed effective uterine contraction with doses as low as 20 μg.
If the obstetrician considered uterine tone adequate at any time intraoperatively, deeming no further requirement for uterotonics, the response to carbetocin treatment was considered a success. In case of unsatisfactory uterine tone and upon the obstetrician’s request for additional uterotonics, oxytocin was started as an intravenous infusion of 40 mU·min−1 as per our institution protocol. If the uterine tone continued to be unsatisfactory, other uterotonics, such as ergonovine and carboprost, were considered as appropriate. If there was an intraoperative demand by the obstetrician for an additional uterotonic, the response to carbetocin treatment was considered a failure.
The primary outcome was the need for additional uterotonic agent intraoperatively. Secondary outcomes included the need for additional uterotonics postoperatively within 24 hr following surgery, estimated blood loss, and side effects, such as hypotension, hypertension, bradycardia, tachycardia, nausea, vomiting, flushing, headache etc., following carbetocin administration. Blood loss was calculated by measuring the hematocrit values before and 24 hr after the CD and using the following formula: EBV × (preoperative hematocrit − postoperative hematocrit) / preoperative hematocrit, where EBV means estimated blood volume (mL), which was calculated as the patient’s body weight × 85.20 Hypotension was defined as a reading of systolic BP < 20% of baseline value despite the prophylactic use of intermittent boluses of phenylephrine, while hypertension was defined as systolic BP > 20% of baseline. Tachycardia and bradycardia were defined as HR either greater than or less than 30% of baseline values, respectively.
Statistical considerations
For dose-finding studies based on the biased-coin up-and-down design, simulation studies suggest that enrolling at least 20-40 patients will provide stable estimates of the target dose for most cases.Footnote 1 , 21,22 Therefore, in this study, we decided to enroll 40 patients. The ED90 and its 95% confidence intervals (CI) were estimated using two non-parametric methods, i.e., the truncated Dixon and Mood method and the isotonic regression method with the pooled-adjacent-violators algorithm approach with linear calibration. Patient characteristics and the secondary outcomes were compared across various dose groups using the Kruskal-Wallis test and the Fisher’s exact test for continuous and categorical variables, respectively. A P value of < 0.05 was considered to be statistically significant. The statistical analyses were performed using SAS® 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
Seventy-two females were approached to participate in the study, and 44 met eligibility criteria and were recruited from June 6 to August 22, 2012. Data analysis was completed for 40 females. Two patients received nitroglycerine prior to uterine incision and hence were excluded as per study protocol. Furthermore, one patient developed intraoperative pulmonary edema prior to the administration of carbetocin (attributed to mitral valve regurgitation, which was diagnosed only postoperatively), and one patient had severe surgical bleeding from dissection of the uterine artery. Both patients had received the allocated drug treatment and had adequate uterine tone after carbetocin; however, due to unrelated intraoperative complications, their results were excluded from the analysis. The details of patient recruitment are presented in Fig. 1.
The demographic and clinical characteristics of the patients according to the dose group are presented in Table 1. The mean (range) age was 37 (24.4-39.8) yr and the mean (range) body mass index was 37 (25-44) kg·m−2.
The patient allocation sequence and response vs dose of carbetocin is shown in Fig. 2. Based on the Dixon-Mood method for non-parametric analysis, the ED90 of carbetocin was 14.8 μg (95% CI 13.7 to 15.8). The ED90 by the isotonic regression estimator method was 13.3 μg.
Thirty-seven (92.5%) females had adequate uterine tone with no requirement of additional intraoperative uterotonics. Three patients who had inadequate uterine tone required additional oxytocin. One patient had received 15 μg of carbetocin and two had received 10 μg (Fig. 2). Two patients who had received carbetocin 15 μg and responded initially with satisfactory uterine contraction later on required oxytocin in the postanesthesia care unit within four hours of carbetocin administration (Table 2).
The mean (SD) estimated blood loss using the hematocrit variation method was 786 (403) mL. The side effects associated with carbetocin administration are shown in Table 3. The overall rates of hypotension and hypertension were 37.5% and 12.5%, respectively, while nausea occurred in 27.5% of patients.
Discussion
For routine elective CD, we found that the ED90 for carbetocin is 14.8 μg (95% CI 13.7 to 15.8). This dose is less than one-fifth of the recommended dose of 100 μg according to the current SOGC guidelines.15
These results further refine two previous dose-finding studies of carbetocin at elective CD conducted at our institution.16,17 Cordovani et al.16 compared carbetocin in doses of 80, 90, 100, 110, and 120 μg and observed similar efficacy in uterine contraction across all groups. Subsequently, Anandakrishnan et al.17 compared carbetocin in doses of 20, 40, 60, 80, and 100 μg and again found similar efficacy for uterine contraction across all treatment groups. The overall success rates of the carbetocin treatments in those studies were 87% and 94.5%, respectively.16,17 Given the monotonic distribution of results in both studies, it was not possible to calculate the ED90; however, those findings involving a total of 200 females suggested that the ED90 could be equal to or lower than 20 μg. That assumption has now been confirmed with the current study.
The effective dose obtained in the current study contrasts with the doses of carbetocin commonly reported and recommended in the literature. In a recent systematic review, Su et al.14 concluded that the risk of PPH in females undergoing CD was lower in the group receiving carbetocin compared with those receiving oxytocin (RR 0.55; 95% CI 0.31 to 0.95). It is noteworthy that all trials included in this systematic review studied carbetocin 100 μg, while the dosage of the oxytocin intravenous bolus varied from 5-32.5 IU over 16 hr.
The fact that we found an ED90 for carbetocin to be considerably lower than the recommended dose may not be all that surprising. Our group has described similar findings for oxytocin. Carvalho et al.18 determined the ED90 of oxytocin at elective CD to be 0.35 IU (95% CI 0.18 to 0.52), almost ten times lower than a routinely recommended 5 IU bolus dose. Butwick et al. have subsequently confirmed these results.23
Our findings may be explained in different ways. The initial recommendation for a 100 μg bolus dose of carbetocin may have originated from animal studies suggesting carbetocin 100 μg is equivalent to oxytocin 5 IU.24 Animal data may not be readily transferable to humans, particularly in the case of carbetocin, as it has been suggested that the human myometrium is much more sensitive to carbetocin than the rat myometrium.24,25 Furthermore, carbetocin exerts its uterotonic effect by binding with oxytocin receptors,11 which have been shown to increase in concentration progressively throughout pregnancy.26 It could thus be postulated that an increased sensitivity of the pregnant uterus leads to effective uterine contraction even at such small doses, as found in our study. It is likely that carbetocin, being a synthetic analogue of oxytocin, displays a similar mechanism of action and dose-response profile as oxytocin.
The primary outcome in the concluding part of this series that led to the ED90 of 14.8 μg (95% CI 3.7 to 15.8) was effective uterine contraction. Given the subjective nature of this outcome, it is important to examine other outcomes that confirm the efficacy of such doses. The mean (SD) estimated blood loss in our current study, 786 (403) mL, is comparable with that seen in the studies by Cordovani et al.,16 649 (427) mL, and Anandakrishnan et al.,17 781 (493) mL, where higher doses of carbetocin ranging from 20-120 μg were used. Therefore, it does not appear that blood loss is affected by decreasing the dose of carbetocin.
Clinical trials to determine the minimum effective dose of oxytocin and carbetocin are justified given the drugs’ dose-dependent side effects, particularly hypotension.7,8,14,16 Although carbetocin shows a longer half-life compared with oxytocin,8,12,13 studies have reported similar side effects, such as hypotension, headache, nausea, vomiting, tremor, shortness of breath, abdominal pain, back pain, and flushing etc.27,28 The incidence of hypotension in our study was 37.5%, less than that reported by Cordovani et al. for a dose range of 80-120 μg of the drug16 and less than the 42.5% incidence shown by Anandakrishnan et al. for a dose range of 20-100 μg.17 Since all these studies were carried out at the same institution with a careful method to standardize diagnosis and treatment of hypotension, we suggest that the incidence of hypotension could be reduced by lowering drug dose while maintaining efficacy. However, none of these studies, including the current one, have been powered to evaluate hypotension.
Our study has several limitations. The relatively small sample size is a result of employing an up-down study methodology. We used a biased-coin up-down design rather than the randomized parallel group design as reported in our previous studies. An advantage of the biased-coin up-down method is that fewer patients are subjected to ineffective or excessive drug doses. Although our previous randomized trials involved 200 subjects, we were unable to determine an ED90 for carbetocin. In retrospect, implementing an up-down methodology from the outset may have led to similar results while recruiting fewer patients. The sampling frame of our initial studies was based on unpublished information from the manufacturer on 18 females undergoing CD, wherein none receiving a dose < 60 μg had effective uterine contractions. Consequently, we could not have anticipated the results of the initial studies, which suggested that a wide range of doses of carbetocin (20-120 μg) can be equally effective.
A second limitation of our study is the subjective nature of assessment of the uterine tone at the time of delivery, which was intrinsically related to our primary outcome. We tried to standardize the process as much as possible by giving a set of instructions to the obstetricians to determine the adequacy of uterine contraction. Despite this limitation, this method has been used in a number of previous studies and ultimately reflects real clinical practice, as it is the method routinely used by obstetricians to guide the administration of uterotonics.
In summary, we have determined the ED90 of carbetocin at elective CD to be 14.8 μg (95% CI 13.7 to 15.8). This dose is less than one-fifth of the recommended dose of 100 μg according to the current SOGC guidelines.
Notes
Stylianou M. Sequential Analysis of Durham and Flournoy’s Biased Coin Design for Phase I Clinical Trials. Washington, DC, American University, Department of Mathematics and Statistics, College of Arts and Sciences; 2000: i-xiii, 1-178.
References
Joseph KS, Rouleau J, Kramer MS, et al. Investigation of an increase in postpartum haemorrhage in Canada. BJOG 2007; 114: 751-9.
Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol 2010; 202: 353.e1-6.
World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: WHO; 2012 Available from: http://apps.who.int/iris/bitstream/10665/75411/1/9789241548502_eng.pdf (accessed October 2013).
Nordstrom L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. Br J Obstet Gynaecol 1997; 104: 781-6.
Ryden G, Sjoholm I. Half-life of oxytocin in blood of pregnant and non-pregnant women. Acta Endocrinol (Copenh) 1969; 61: 425-31.
Chard T, Boyd NR, Forsling ML, McNeilly AS, Landon J. The development of a radioimmunoassay for oxytocin: the extraction of oxytocin from plasma, and its measurement during parturition in human and goat blood. J Endocrinol 1970; 48: 223-34.
Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Curr Opin in Anaesthesiol 2011; 24: 255-61.
Dansereau J, Joshi AK, Helewa ME, et al. Double-blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. Am J Obstet Gynecol 1999; 180(3 Pt 1): 670-6.
Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth 2008; 100: 683-9.
Engstrom T, Barth T, Melin P, Vilhardt H. Oxytocin receptor binding and uterotonic activity of carbetocin and its metabolites following enzymatic degradation. Eur J Pharmacol 1998; 355: 203-10.
Sweeney G, Holbrook AM, Levine M, et al. Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, in nonpregnant women. Curr Ther Res 1990; 47: 528-40.
Attilakos G, Psaroudakis D, Ash J, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG 2010; 117: 929-36.
Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum hemorrhage after cesarean section: a randomized clinical trial. Arch Gynecol Obstet 2009; 280: 707-12.
Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012; 4: CD005457.
Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2009; 31: 980-93.
Cordovani D, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose. Can J Anesth 2012; 59: 751-7.
Anandakrishnan S, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose, part 2. Can J Anesth 2013; . DOI:10.1007/s12630-013-0028-2.
Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose finding study. Obstet Gynecol 2004; 104: 1005-10.
Balki M, Ronayne M, Davies S, et al. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstet Gynecol 2006; 107: 45-50.
Shook PR, Schultz JR, Reynolds JD, Barbara P, Spahn TE, DeBalli P. Estimating blood loss for cesarean section – how accurate are we? Anesthesiology 2003; 98(Supp 1): SOAP A2 (abstract).
Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 2007; 107: 144-52.
Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics 2002; 58: 171-7.
Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective caesarean delivery. Br J Anaesth 2010; 104: 338-43.
Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long acting oxytocin analogue on the postpartum uterus. Clin Pharmacol Ther 1992; 52: 60-7.
Atke A, Vilhardt H. Uterotonic activity and myometrial receptor affinity of 1-deamino-1-carba-2-tyrosine(O-methyl)-oxytocin. Acta Endocrinol (Copenh) 1987; 115: 155-60.
Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature 1992; 356: 526-9.
Peters NC, Duvekot JJ. Carbetocin for the prevention of postpartum hemorrhage: a systematic review. Obstet Gynecol Surv 2009; 64: 129-35.
Boucher M, Horbray GL, Griffin P, et al. Double-blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. J Perinatol 1998; 18: 202-7.
Acknowledgements
The authors acknowledge the following contributions: Kristi Downey, MSc, Perinatal Research Coordinator at the Department of Anesthesia and Pain Management, Mount Sinai Hospital, for her invaluable help and support in all stages of this study; Xiang Y Ye, MSc, Maternal-Infant Care Research Centre, Mount Sinai Hospital, Toronto, ON, Canada for the statistical analysis.
Funding
Departmental.
Conflict of interest
Dan Farine serves as a consultant for Ferring Pharmaceutics.
Disclosures
This study won the Richard Knill Best Paper at the 2013 Annual Competition of the Canadian Anesthesiologists’ Society and 2nd place in the Gertie Marx Competition at the 45th Annual Meeting of the Society for Obstetric Anesthesia and Perinatology, San Juan, Puerto Rico, April 25-28, 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author’s contributions
Mubeen Khan, Mrinalini Balki, Dan Farine, Gareth Seaward, and Jose C.A. Carvalho participated in the research design. Mubeen Khan, Mrinalini Balki, Iram Ahmed, and Jose C.A. Carvalho participated in data collection. Mubeen Khan, Mrinalini Balki, Dan Farine, and Jose C.A. Carvalho participated in data interpretation. Mubeen Khan, Mrinalini Balki, and Jose C.A. Carvalho participated in manuscript writing.
Rights and permissions
About this article
Cite this article
Khan, M., Balki, M., Ahmed, I. et al. Carbetocin at elective Cesarean delivery: a sequential allocation trial to determine the minimum effective dose. Can J Anesth/J Can Anesth 61, 242–248 (2014). https://doi.org/10.1007/s12630-013-0082-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0082-9