Abstract
Purpose
The aim of this study was to determine the intravenous dose of carbetocin required to produce effective uterine contraction in 95% of women (ED95) undergoing elective Cesarean delivery under spinal anesthesia.
Methods
One hundred and twenty term pregnant women at low risk for postpartum hemorrhage (PPH) undergoing elective Cesarean delivery under spinal anesthesia were randomly allocated to receive carbetocin in doses of 20, 40, 60, 80, or 100 μg iv upon delivery of the fetus. The obstetrician evaluated the efficacy of uterine tone as satisfactory or unsatisfactory, and in case of unsatisfactory tone, additional uterotonics were administered as per routine institutional practice. The primary outcome measure was satisfactory uterine tone at two minutes after carbetocin administration, and the secondary outcomes were the estimated blood loss, need for additional uterotonic agents within 24 hr, and side effects.
Results
Overall satisfactory uterine tone at two minutes was observed in 94.2% (113/120) of the women, and there was no difference across the different study groups. It was not possible to calculate the ED95 of carbetocin due to the even distribution of women with unsatisfactory uterine tone at two minutes across all dose groups (P = 0.60). Additional uterotonics within 24 hr were required in 13% (16/120) of the women. Side effects were similar across all dose groups, with an overall 42.5% incidence of hypotension following the administration of carbetocin.
Conclusions
In women at low risk for PPH undergoing elective Cesarean delivery under spinal anesthesia, carbetocin is similarly effective in doses of 20-100 μg. There is a high incidence of hypotension associated with carbetocin in these doses. Further dose-finding studies are warranted, including doses lower than 20 μg. This trial was registered at www.clinicaltrials.gov (NCT01428817).
Résumé
Objectif
L’objectif de cette étude était de déterminer la dose intraveineuse de carbétocine nécessaire à produire une contraction utérine efficace chez 95 % des femmes (DE95) subissant un accouchement par césarienne programmé sous rachianesthésie.
Méthode
Cent vingt femmes enceintes à terme et présentant un faible risque d’hémorragie postpartum (HPP) subissant un accouchement programmé par césarienne sous rachianesthésie ont reçu de façon aléatoire de la carbétocine à des doses de 20, 40, 60, 80 ou 100 μg lors de la délivrance du fœtus. L’obstétricien a évalué l’efficacité du tonus utérin comme étant satisfaisante ou insatisfaisante et, en cas de tonus insatisfaisant, des agents utérotoniques supplémentaires ont été administrés selon la pratique habituelle de l’institution. Le critère d’évaluation principal était un tonus utérin satisfaisant deux minutes après l’administration de carbétocine, et les critères secondaires étaient la perte de sang estimée, le besoin d’agents utérotoniques supplémentaires au cours des 24 premières heures, et les effets secondaires.
Résultats
Un tonus utérin globalement satisfaisant à deux minutes a été observé chez 94,2 % (113/120) des femmes, et il n’y a pas eu de différence entre les divers groupes à l’étude. Il n’a pas été possible de calculer la DE95 de la carbétocine en raison de la distribution égale des femmes présentant un tonus utérin insatisfaisant à deux minutes dans tous les groupes de doses (P = 0,60). Les effets secondaires étaient semblables dans tous les groupes, avec une incidence globale de 42,5 % d’hypotension suivant l’administration de carbétocine.
Conclusion
Chez les femmes courant un risque faible d’HPP et subissant un accouchement par césarienne programmé sous rachianesthésie, la carbétocine est aussi efficace à des doses de 20-100 μg. Il y a une forte incidence d’hypotension associée à la carbétocine à de telles doses. D’autres études d’évaluation de doses sont nécessaires, y compris avec des doses plus basses que 20 μg. Cette étude a été enregistrée au www.clinicaltrials.gov (NCT01428817).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Postpartum hemorrhage (PPH) remains one of the leading causes of maternal morbidity and mortality worldwide.1 Uterine atony has been increasingly implicated as the main cause of PPH,2,3 and as such, the World Health Organization recommends active management of the third stage of labour to prevent PPH, even in low-risk patients.4 Given that the most common cause of PPH is uterine atony, not surprisingly, uterotonics are the first line of drugs selected to prevent and treat PPH.
Oxytocin is the most commonly used uterotonic worldwide. Nevertheless, when the Society of Obstetricians and Gynaecologists of Canada (SOGC) revisited its guidelines for the management of PPH in 2009, it recommended using carbetocin to replace oxytocin as the uterotonic of choice at elective Cesarean deliveries. The recommended dose was 100 μg injected intravenously over one minute.5
Carbetocin (1-deamino-1-carba-2-tyrosine (0-methyl)-oxytocin) is a newer uterotonic agent. On the one hand, it is an oxytocin derivative that binds to the oxytocin receptor in the myometrium with a similar affinity to its parent drug, but on the other hand, its half-life of elimination is approximately 40 min after intravenous injection, which is four to ten times longer than that of oxytocin.6 This accounts for its protracted uterotonic activity providing the advantage of a single bolus dosing rather than a continuous infusion as with oxytocin. In a recent systematic review,7 carbetocin has been shown to reduce the need for additional uterotonics when compared with oxytocin. This may have been the motivation behind the SOGC’s recommendation.
Although the dose of 100 μg has been used in most clinical trials to date7 and is now recommended by the SOGC,5 our group recently conducted a dose-finding study of carbetocin at elective Cesarean deliveries with doses of 80, 90, 100, 110, and 120 μg. The overall incidence of effective uterine contraction was 87%, and we observed no significant differences in the response rate across the studied groups.8 Furthermore, we did not observe significant differences in blood loss across the studied groups. The overall incidence of hypotension associated with the use of carbetocin was high at 55%, and this was also similar across all groups. While we were unable to determine the ED95 of carbetocin, the findings of our recent study suggest that the effective dose of carbetocin could be lower than the currently recommended dose of 100 μg.
The purpose of this study was to determine the dose of carbetocin to produce effective uterine contraction in 95% (ED95) of women undergoing elective Cesarean delivery under spinal anesthesia.
Methods
Following approval by the Mount Sinai Hospital Research Ethics Board (Approval 11-0128-E, approval date June 16, 2011), this study was conducted at the Mount Sinai Hospital, Toronto, Canada as a randomized double-blind dose-finding study. Written informed consent was obtained from all participants. The study was conducted from June to December 2011.
The eligible population consisted of healthy women at low risk for PPH scheduled for elective Cesarean delivery under spinal anesthesia. Exclusion criteria included women with American Society of Anesthesiologists’ physical status III and above, those requiring general anesthesia, and those at high risk for PPH, including multiple pregnancy, presence of fibroids, polyhydramnios, macrosomia, previous uterine atony or PPH, abnormal placentation, or known coagulopathy and/or bleeding diathesis.
Women who agreed to participate were randomly allocated into five groups to receive carbetocin in doses of 20, 40, 60, 80, or 100 μg. Randomization was performed by computer in blocks of ten. The dose was printed on paper, placed in an opaque envelope, and labelled only with the study patient number. The study drug used was the commercially available carbetocin in an ampoule containing 100 μg·mL−1. It was stored in the refrigerator in accordance with the manufacturer’s storage guidelines and prepared immediately before use by an independent research assistant. The study drug was drawn with a 1 mL syringe divided into 0.1 mL gradations. It was then made up with saline to 10 mL using a 10-mL syringe and labelled to reveal only the study patient number. The investigator collecting the data, the anesthesiologist, the surgeon, and the patient were all blinded to the dose of carbetocin.
Baseline blood pressure (BP) and heart rate were taken in the pre-assessment area and averaged over three readings, each measured five minutes apart. Hemoglobin and hematocrit were measured routinely immediately preoperatively in blood samples collected during intravenous insertion of an 18G cannula.
Monitoring consisted of electrocardiogram, noninvasive BP, and oxygen saturation using pulse oximetry as per our standard practice. All patients received a coload with 10 mL·kg−1 of Ringer’s lactate concomitantly with the placement of spinal anesthesia. After the initial fluid coload, the rate of fluid infusion was decreased to 125 mL·hr−1. Spinal anesthesia was performed in the sitting position at the level of the L2/3 or L3/4 interspace. The spinal anesthetic mixture was comprised of 0.75% bupivacaine 1.8 mL, fentanyl 10 μg, and morphine 100 μg. Analgesic adjuncts, such as ketorolac 30 mg iv and acetaminophen 1.3 g per rectum, were administered intraoperatively after fetal delivery.
Blood pressure was taken every minute, continued at the same interval after delivery for five minutes, and then the frequency was reduced to every 2.5 min. We aimed at maintaining systolic blood pressure (SBP) at baseline levels pre and post delivery with the use of prophylactic boluses of phenylephrine. Any drop in SBP below baseline levels was treated with phenylephrine 100 μg. Hypotension was defined as a decrease in SBP > 20% below baseline despite the prophylactic use of phenylephrine, in which case a rescue dose of phenylephrine 200 μg was used. Hypertension was defined as an increase in SBP > 20% above baseline. Tachycardia and bradycardia were defined as a 30% increase or decrease, respectively, in baseline heart rate.
The 10 mL study solution of carbetocin was administered over one minute by the anesthesiologist immediately upon delivery of the fetal head. The assessment of uterine tone was performed by the obstetrician at one minute intervals from two minutes after completion of carbetocin administration. They rated the uterine tone as “firm” (satisfactory) or “boggy” (unsatisfactory). Unsatisfactory tone was determined as that which would require medical intervention if unchanged for two minutes. Placental delivery was performed by controlled cord traction alone with no initial uterine massage applied. At any time after two minutes post administration of carbetocin, the obstetrician (after attempting uterine massage) could request “rescue” oxytocin at our regular dose. This comprised a bolus of oxytocin 0.5 IU followed by an infusion of oxytocin 20 IU·L−1 at a rate of 125 mL·hr−1. If required, ergot or carboprost could also be administered.
Patients’ complaints of nausea, vomiting, headache, dyspnea, chest pain, palpitations, and observed flushing were recorded. Postoperative hemoglobin and hematocrit values were obtained 24 hr post Cesarean delivery to estimate blood loss (EBL), which was calculated according to the following formula:
where EBV is the estimated blood volume (mL), calculated as the patient’s weight (kg) × 85 mL.Footnote 1
The primary outcome of our study was to determine the intravenous dose of carbetocin required to produce effective uterine contractions in 95% of women (ED95) at two minutes after carbetocin administration. Secondary outcomes included the use of additional uterotonics within 24 hr, estimated blood loss, and side effects attributed to carbetocin.
Sample size
Sample size calculations were implemented through the MCPMod package in R for the multiple comparison procedure and modelling approach to dose-finding studies.9 Sample size was calculated based on finding the clinically effective dose—defined as the dose required to achieve effective uterine tone in 95% of treated individuals (ED95)—using the methods derived by Bretz et al. for dose-finding studies.10 Data on 80 subjects from our previous study8 showing an 87.5% response with carbetocin doses from 80-120 μg were used to inform the sample size calculation. Using data from Cordovani et al. 8 and from Ferring Pharmaceutics (unpublished data on 18 women undergoing elective Cesarean delivery wherein none had effective uterine contractions with doses below 60 μg, and 83% [five out of six] developed adequate uterine tone after receiving a dose of 100 μg), a simple logistic dose-response curve was deemed the most likely model out of several candidate curves. Additionally, a two-tailed type I error of 0.05 and a type II error of 0.10 (i.e., power of 90%) were assumed. Under these assumptions and equal sample sizes in each of the five dose groups, 21 women per group would be required to determine the ED95. To account for a 15% potential loss to follow-up/failure to complete the protocol, 24 women per group were recruited for a total of 120 women.
Data analysis
To address the primary aim of finding the ED95 of carbetocin at two minutes, we planned to use the multiple comparison procedure and modelling approach introduced by Bretz et al. 11 Nevertheless, due to very few incidents of inadequate uterine tone, this approach could not be used and the ED95 could not be determined. Fisher’s exact tests were used to compare differences in categorical outcomes across treatment groups, and one-way analysis of variance was used to compare continuous outcomes across treatment groups. A step-down Bonferroni correction for multiple comparisons was to be conducted for any post hoc comparisons. All analyses were conducted using SAS® version 9.2 (Cary, NC, USA). All tests were two-sided, and P values < 0.05 were considered statistically significant.
Results
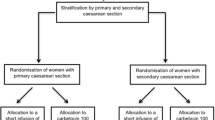
Of the 183 women who were approached to participate in the study, 41 declined to participate and 22 did not meet the inclusion criteria. The remaining 120 women were randomized to five different dosing groups (i.e., 20, 40, 60, 80, and 100 μg) with 24 subjects in each group (Figure).
Patient characteristics across the different groups are presented in Table 1.
Effective uterine contraction at two minutes was observed in 94.2% (113/120) of women and did not differ significantly across the study groups (P = 0.60) (Table 2); therefore, it was not possible to create a dose-response curve to calculate the ED95 of carbetocin, which was the primary aim of this study.
There was no difference in the requirement for additional uterotonics within 24 hr across the study groups (P = 0.30) (Table 2). All women requiring additional uterotonics (16/120) did so within the first four hours post delivery. In all cases, an oxytocin infusion was started, and no ergot or carboprost was used. There was no difference in estimated blood loss across the studied groups (P = 0.53) (Table 2).
The incidence of side effects attributed to carbetocin is shown in Table 3. The most common side effect was hypotension, with an overall incidence of 42.5%. There was no difference in phenylephrine usage after the administration of carbetocin across the study groups (Table 4).
Discussion
Our results show that carbetocin doses of 20, 40, 60, 80, and 100 μg are equally effective at promoting effective uterine contraction in women at low risk for PPH undergoing elective Cesarean delivery. Furthermore, due to the monotonic nature of our results, it was not possible to create a dose-response curve to determine the ED95 for carbetocin.
It remains unclear how the dose of 100 μg was initially recommended. In vitro studies on myometrial strips from pregnant or estrogen-primed rats showed carbetocin possessing one-thirtieth the contractile activity of oxytocin,12,13 whereas in vivo studies on the uterus of rats showed carbetocin possessing one-tenth the activity of oxytocin.12,14 This led Hunter et al. to assume that 100 μg of carbetocin would produce the same effect as 10 μg (equivalent to 5 IU) of oxytocin.15 It is important to highlight, however, that this information has been derived from animal data, and it is not clear whether the potency ratio found in rat models can be extrapolated to humans. Assuming that these potency ratios are correct, our results are not surprising. The use of doses higher than necessary has also been reported with oxytocin. While many protocols in the past have recommended doses of 5-10 IU iv at elective Cesarean deliveries, our own group has shown that the ED90 of oxytocin at elective Cesarean deliveries is only 0.35 IU (95% confidence interval, 0.18 to 0.52).16 It is therefore possible that the effective dose of carbetocin is much lower than that currently recommended.
Given the subjective nature of the assessment of our primary outcome, we considered it important to look at the need for additional uterotonics and the estimated blood loss as surrogates for the efficacy of carbetocin. Our results are in keeping with those of Cordovani et al.8 whereby the need for additional uterotonics and the overall blood loss are similar and did not vary across the study groups. Such results further reinforce that carbetocin doses in the range of 20-120 μg are similarly effective in this patient population.
Carbetocin binds to oxytocin receptors in the myometrium in a manner similar to oxytocin. It is therefore acceptable to assume that it will bind to oxytocin receptors located in the cardiovascular system in a similar fashion and determine cardiovascular effects similar to those of oxytocin. Oxytocin, even in small doses, has been shown to promote dose-dependent cardiovascular effects, notably hypotension.17 It is therefore important to use the minimum effective dose. The incidence of hypotension in this study (42.5%) was high and similar to that observed by Cordovani et al. (55%).8 The amount of phenylephrine used as well as the incidence of nausea and vomiting did not vary across the studied groups, suggesting that the hemodynamic consequences of carbetocin are similar in this dose range. It is unclear whether reducing the administered dose of carbetocin may further reduce the incidence of this unwanted effect; therefore, further studies are warranted. Furthermore, although it is well established that oxytocin given by infusion produces fewer hemodynamic effects than when given by bolus,18 less is known about the method of administration of carbetocin. It remains unclear why the manufacturer has recommended administration of carbetocin as an intravenous bolus over one minute. Further studies are needed to investigate whether a slow infusion of carbetocin provides more cardiovascular stability, as is the case with oxytocin, though this clearly detracts from the convenience of its current method of administration.
Our study has some limitations. The assessment of uterine tone by the obstetrician, our primary outcome, is subjective. Nevertheless, a similar method is frequently used in the literature, as it reflects how uterotonics are used in real clinical practice. Our study did not include a placebo group in which no uterotonic was used. Although the inclusion of a placebo group would have been ideal, we did not consider it ethically acceptable, as the administration of uterotonics as part of active management of the third stage is considered a standard of practice. Assessment of the estimated blood loss according to the change in hematocrit lacks validation against a more robust method and is subject to criticism.
In summary, carbetocin doses of 20-100 μg are similarly effective in women at low risk for PPH undergoing elective Cesarean deliveries. The incidence of side effects associated with these doses, notably hypotension, remains high and similar across all doses. Further dose-finding studies, including doses lower than 20 μg, are warranted to determine efficacy and side effects.
Notes
Shook PR, Schultz JR, Reynolds JD, Spahn TE, DeBalli P. Estimating blood loss for cesarean section—how accurate are we? Anesthesiology 2003; 98(Supp 1): SOAP A2.
References
Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. Br J Obstet Gynaecol 2011; 118(Suppl 1): 1-203.
Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth 2009; 9: 55.
Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg 2010; 110: 1368-73.
World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: WHO; 2012 Available from URL: http://apps.who.int/iris/bitstream/10665/75411/1/9789241548502_eng.pdf (accessed April 2013).
Leduc D, Senikas V, Lalonde AB, Clinical Practice Obstetrics Committee, Society of Obstetricians and Gynaecologists of Canada, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2009; 31: 980-93.
Sweeney G, Holbrook AM, Levine M, et al. Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, in non-pregnant women. Curr Ther Res 1990; 47: 528-40.
Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012; 4: CD005457.
Cordovani D, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to find the effective dose. Can J Anesth 2012; 59: 751-7.
Bornkamp B, Pinheiro J, Bretz F. MCPMod: An R package for the design and analysis of dose finding studies. J Stat Software 2009; 29: 1-23.
Bretz F, Dette H, Pinheiro JC. Practical considerations for optimal designs in clinical dose finding studies. Stat Med 2010; 29: 731-42.
Bretz F, Pinheiro JC, Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics 2005; 61: 738-48.
Barth T, Jost K, Rychlik I. Milk-ejecting and uterotonic activities of oxytocin analogues in rats. Endocrinol Exp 1975; 9: 35-42.
Atke A, Vilhardt H. Uterotonic activity and myometrial receptor affinity of 1-deamino-1-carba-2-tyrosine(O-methyl)-oxytocin. Acta Endocrinol (Copenh) 1987; 115: 155-60.
Barth T, Slaninova J, Lebl M, Jost K. Biological activities and protracted action of carba-analogues of deamino-oxytocin with o-methyltyrosine in position 2. Coll Czech Chem Commun 1980; 45: 3045-50.
Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clin Pharmacol Ther 1992; 52: 60-7.
Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol 2004; 104: 1005-10.
Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective caesarean delivery. Br J Anaesth 2010; 104: 338-43.
Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. Br J Anaesth 2007; 98: 116-9.
Acknowledgements
We sincerely thank Mrs. Kristi Downey MSc, Perinatal Research Coordinator at the Department of Anesthesia and Pain Management, Mount Sinai Hospital, for her invaluable contribution in recruiting patients and for creating and maintaining the database. We also thank J. Charles Victor, epidemiologist at the Institute for Clinical Evaluative Sciences, for his support with statistical analysis.
Funding
Departmental.
Conflict of interest
Dan Farine serves as a consultant for Ferring Pharmaceutics.
Disclosure
This paper was presented, in part, at the 44th Annual Meeting of the Society for Obstetric Anesthesia and Perinatology, Monterey, CA, May 2-5, 2012 and won the Zuspan Award. The paper was also presented at the Canadian Anesthesiologists’ Society Annual Meeting, Quebec City, QC, June 15-18, 2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2013; 60: this issue.
Author contributions
Suresh Anandakrishnan, Mrinalini Balki, Dan Farine, Gareth Seaward, and Jose C.A. Carvalho participated in designing the research and collecting and interpreting the data. Suresh Anandakrishnan and Jose C.A. Carvalho participated in writing the manuscript. Dan Farine, Gareth Seaward and Mrinalini Balki reviewed the manuscript. Jose C.A. Carvalho submitted the final version of the manuscript and is the author responsible for keeping the research files.
Rights and permissions
About this article
Cite this article
Anandakrishnan, S., Balki, M., Farine, D. et al. Carbetocin at elective Cesarean delivery: a randomized controlled trial to determine the effective dose, part 2. Can J Anesth/J Can Anesth 60, 1054–1060 (2013). https://doi.org/10.1007/s12630-013-0028-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0028-2