Abstract
Purpose
Postpartum hemorrhage (PPH) is a leading cause of maternal mortality worldwide. Although several studies on the prophylactic use of tranexamic acid (TXA) in parturients undergoing Cesarean delivery have been published, conflicting results raise questions regarding its use. Thus, we aimed to investigate the safety and efficacy of PPH prophylaxis with TXA.
Source
We searched PubMed®, Embase, Cochrane Central, and ClinicalTrials.gov for randomized controlled trials (RCTs) comparing prophylactic TXA with placebo or no treatment in parturients undergoing Cesarean delivery. Our main outcomes were PPH, any blood transfusion, need for additional uterotonics, and adverse events. We performed a trial sequential analysis (TSA) of all outcomes to investigate the reliability and conclusiveness of findings.
Principal findings
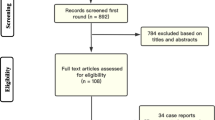
We included 38 RCTs including 22,940 parturients, 11,535 (50%) of whom were randomized to receive prophylactic TXA. Patients treated with TXA had significantly fewer cases of PPH (risk ratio [RR], 0.51; 95% confidence interval [CI], 0.38 to 0.69; P < 0.001); less blood transfusion (RR, 0.43; 95% CI, 0.30 to 0.61; P < 0.001), and less use of additional uterotonics (RR, 0.52; 95% CI, 0.40 to 0.68; P < 0.001). No significant differences were found between the groups in terms of adverse effects and thromboembolic events.
Conclusion
Prophylactic TXA administration for parturients undergoing Cesarean delivery significantly reduced blood loss, without increasing adverse events, supporting its use as a safe and effective strategy for reducing PPH in this population.
Study registration
PROSPERO (CRD42023422188); first submitted 27 April 2023.
Résumé
Objectif
L’hémorragie du post-partum (HPP) est l’une des principales causes de mortalité maternelle dans le monde. Bien que plusieurs études sur l’utilisation prophylactique d’acide tranexamique (TXA) chez les personnes parturientes ayant accouché par césarienne aient été publiées, des résultats contradictoires soulèvent des questions quant à son utilisation. Ainsi, nous avons cherché à étudier l’innocuité et l’efficacité de la prophylaxie à base de TXA pour l’HPP.
Sources
Nous avons fait une recherche sur PubMed®, Embase, Cochrane Central et ClinicalTrials.gov pour en tirer les études randomisées contrôlées (ERC) comparant le TXA prophylactique à un placebo ou à l’absence de traitement chez les personnes parturientes accouchant par césarienne. Nos principaux critères d’évaluation étaient l’HPP, toute transfusion sanguine, la nécessité d’un utérotonique supplémentaire et les événements indésirables. Nous avons effectué une analyse séquentielle des études pour tous les résultats afin d’examiner la fiabilité et le caractère concluant des conclusions.
Constatations principales
Nous avons inclus 38 ERC comprenant 22 940 personnes parturientes, dont 11 535 (50 %) ont été randomisées pour recevoir du TXA prophylactique. La patientèle traitée par TXA présentait significativement moins de cas d’HPP (risque relatif [RR], 0,51; intervalle de confiance [IC] à 95 %, 0,38 à 0,69; P < 0,001); moins de transfusion sanguine (RR, 0,43; IC 95 %, 0,30 à 0,61; P < 0,001) et moins d’utilisation d’utérotoniques supplémentaires (RR, 0,52; IC 95 %, 0,40 à 0,68; P < 0,001). Aucune différence significative n’a été constatée entre les groupes en termes d’effets indésirables et d’événements thromboemboliques.
Conclusion
L’administration prophylactique de TXA pour les personnes parturientes accouchant par césarienne a considérablement réduit les pertes de sang sans augmenter les événements indésirables, ce qui soutient son utilisation comme stratégie sécuritaire et efficace pour réduire l’HPP dans cette population.
Enregistrement de l’étude
PROSPERO (CRD42023422188); première soumission le 27 avril 2023.






Similar content being viewed by others
References
Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006; 367: 1066–74. https://doi.org/10.1016/s0140-6736(06)68397-9
Calvert C, Thomas SL, Ronsmans C, Wagner KS, Adler AJ, Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLoS One 2012; 7: e41114. https://doi.org/10.1371/journal.pone.0041114
Escobar MF, Nassar AH, Theron G, et al. FIGO recommendations on the management of postpartum hemorrhage 2022. Int J Gynaecol Obstet 2022; 157: 3–50. https://doi.org/10.1002/ijgo.14116
Gallos I, Devall A, Martin J, et al. Randomized trial of early detection and treatment of postpartum hemorrhage. N Engl J Med 2023: 389: 11–21. https://doi.org/10.1056/nejmoa2303966
Cai J, Ribkoff J, Olson S, et al. The many roles of tranexamic acid: an overview of the clinical indications for TXA in medical and surgical patients. Eur J Haematol 2020; 104: 79–87. https://doi.org/10.1111/ejh.13348
Simonazzi G, Bisulli M, Saccone G, Moro E, Marshall A, Berghella V. Tranexamic acid for preventing postpartum blood loss after Cesarean delivery: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand 2016; 95: 28–37. https://doi.org/10.1111/aogs.12798
Wang Y, Liu S, He L. Prophylactic use of tranexamic acid reduces blood loss and transfusion requirements in patients undergoing Cesarean section: a meta‐analysis. J Obstet Gynaecol Res 2019; 45: 1562–75. https://doi.org/10.1111/jog.14013
Bellos I, Pergialiotis V. Tranexamic acid for the prevention of postpartum hemorrhage in women undergoing Cesarean delivery: an updated meta-analysis. Am J Obstet Gynecol 2022; 226: 510–23. https://doi.org/10.1016/j.ajog.2021.09.025
Pacheco LD, Clifton RG, Saade GR, et al. Tranexamic acid to prevent obstetrical hemorrhage after Cesarean delivery. N Engl J Med 2023; 388: 1365–75. https://doi.org/10.1056/nejmoa2207419
Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 2021; 134: 103–12. https://doi.org/10.1016/j.jclinepi.2021.02.003
Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed. Chichester: John Wiley & Sons; 2019.
Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–6. https://doi.org/10.1016/j.jclinepi.2010.07.015
Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008; 61: 64–75. https://doi.org/10.1016/j.jclinepi.2007.03.013
Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA); 2017. Available from URL: https://ctu.dk/wp-content/uploads/2021/03/2017-10-10-TSA-Manual-ENG_ER.pdf (accessed November 2023).
Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, Menoufy M, Gülmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective Cesarean section: randomized clinical trial. J Matern Fetal Neonatal Med 2013; 26: 1705–9. https://doi.org/10.3109/14767058.2013.794210
Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective Caesarean delivery. J Matern Fetal Neonatal Med 2015; 28: 1014–8. https://doi.org/10.3109/14767058.2014.941283
Bharati K, Bhuvneshwar K, Bharati S. A double blinded randomized controlled study to evaluate the outcome of IV tranexamic acid versus topical tranexamic acid application in prevention of postpartum hemorrhage in women with placenta previa. Int J Pharm Clin Res 2022; 14: 162–71. Available from URL: http://impactfactor.org/PDF/IJPCR/14/IJPCR,Vol14,Issue2,Article24.pdf (accessed November 2023).
Bhavana G, Abishek MV, Mittal S. Efficacy of prophylactic tranexamic acid in reducing blood loss during and after Caesarean section. Int J Reprod Contracept Obstet Gynecol 2016: 5: 2011–6. https://doi.org/10.18203/2320-1770.ijrcog20161708
Chaiyakarn S, Lerthiranwong T. Efficacy of preoperative intravenous tranexamic acid before Cesarean section in placenta previa: a randomized double blind control trial. J Med Assoc Thai 2023; 106: 235–43.
Abd El-Gaber AEG, Ahmed HH, Khodry MM, Abbas AM. Effect of tranexamic acid in prevention of postpartum hemorrhage in elective caesarean delivery: a randomized controlled study. Int J Reprod Contracept Obstet Gynecol 2019; 8: 1–5. https://doi.org/10.18203/2320-1770.ijrcog20185401
El-Sttar MM, El-Gayed A, Dawood R, El-Sayd Ghnnam Y. Misoprostol and tranexamic acid role in reducing blood loss during the elective Cesarean section. Menoufia Med J 2019; 32: 465.
Gai M, Wu L, Su Q, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after Caesarian section: a multi-center, randomized trial. Eur J Obstet Gynecol Reprod Biol 2004; 112: 154–7. https://doi.org/10.1016/s0301-2115(03)00287-2
Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment Cesarean section: a double-blind randomized case control prospective trial. Saudi J Anaesth 2013; 7: 427–31. https://doi.org/10.4103/1658-354x.121077
Gungorduk K, Yıldırım G, Asıcıoğlu O, Gungorduk O, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective Cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol 2011; 28: 233–40. https://doi.org/10.1055/s-0030-1268238
Gwanzura C. Tranexamic acid versus no tranexamic acid for the prevention of postpartum haemorrhage among women undergoing elective caesarean section at two hospitals in Harare, Zimbabwe: a randomised controlled trial. NCT04733157 2023.
Halifa I, Olusesan Oluwasola T, Fawole B, Oladokun A. Intravenous tranexamic acid for reducing blood loss during Cesarean delivery: a double-blind, randomized-controlled trial. N Niger J Clin Res 2021; 10:40.
Ifunanya NJ, Chukwu IC, Nobert OC, Blessing O, Chibuzor UD, Uchenna OV. Tranexamic acid versus placebo for prevention of primary postpartum haemorrhage among high risk women undergoing Caesarean section in Abakaliki: a randomized controlled trial. Open J Obstet Gynecol 2019; 9: 914–22. https://doi.org/10.4236/ojog.2019.96089
Jafarbegloo E, Faridnyia F, Ahmari Tehran H. The impact of intravenous tranexamic acid on hemoglobin and hematocrit levels after Cesarean delivery in women at low risk for postpartum hemorrhage: a randomized controlled trial. J Midwifery Reprod Health 2022; 10; 1–7.
Kafayat H, Janjua M, Naheed I, Iqbal T. To assess the prophylactic role of tranexamic acid in reducing blood loss during and after two hours of Caesarean section. Pak J Med Health Sci 2018; 12: 1662–5.
Kamel HE, Farhan AM, Abou Senna HF, Khedr MA, Albhairy AA. Role of prophylactic tranexamic acid in reducing blood loss during elective Caesarean section in rural area. Egypt J Hosp Med 2018; 73: 6886–96. https://doi.org/10.21608/ejhm.2018.16939
Lakshmi SD, Abraham R. Role of prophylactic tranexamic acid in reducing blood loss during elective Caesarean section: a randomized controlled study. J Clin Diagn Res 2016; 10: QC17–21. https://doi.org/10.7860/jcdr/2016/21702.9050
Lee SH, Kwek ME, Tagore S, et al. Tranexamic acid, as an adjunct to oxytocin prophylaxis, in the prevention of postpartum haemorrhage in women undergoing elective caesarean section: a single‐centre double‐blind randomised controlled trial. BJOG 2023: 130: 1007–15. https://doi.org/10.1111/1471-0528.17445
Maged AM, Helal OM, Elsherbini MM, et al. A randomized placebo-controlled trial of preoperative tranexamic acid among women undergoing elective Cesarean delivery. Int J Gynaecol Obstet 2015; 131: 265–8. https://doi.org/10.1016/j.ijgo.2015.05.027
Milani F, Haryalchi K, Sharami SH, Atrkarroshan Z, Farzadi S. Prophylactic effect of tranexamic acid on hemorrhage during and after the Cesarean section. Int J Women’s Health Reprod Sci 2018; 7: 74–8. https://doi.org/10.15296/ijwhr.2019.12
Naeiji Z, Delshadiyan N, Saleh S, Moridi A, Rahmati N, Fathi M. Prophylactic use of tranexamic acid for decreasing the blood loss in elective Cesarean section: a placebo-controlled randomized clinical trial. J Gynecol Obstet Hum Reprod 2021; 50: 101973. https://doi.org/10.1016/j.jogoh.2020.101973
Nargis N, Farhana D. Prophylactic use of tranexamic acid during Caesarean section in preventing postpartum haemorrhage—a prospective randomised double blind placebo controlled study. Bangladesh J Obstet Gynaecol 2018; 33: 125–30.
Obi VO, Umeora, Dimejesi, Asiegbu, Mgbafulu, Ifemelumma, Obi. Efficacy of intravenous tranexamic acid at reducing blood loss during elective caesarean section in Abakaliki: a double blind randomized placebo controlled trial. African Journal of Medical and Health Sciences 2019; 18:10–7.
Ogunkua OT, Duryea EL, Nelson DB, et al. Tranexamic acid for prevention of hemorrhage in elective repeat Cesarean delivery—a randomized study. Am J Obstet Gynecol MFM 2022; 4: 100573. https://doi.org/10.1016/j.ajogmf.2022.100573
Omawumi D, Oranu E, Ogu R, Orazulike N, Otokwala J. Effect of intravenous tranexamic acid in reducing blood loss during and after elective Caesarean section in a third level health institution: a randomized controlled study. Open J Obstet Gynecol 2023; 13: 265–79. https://doi.org/10.4236/ojog.2023.132028
Oseni RO, Zakari M, Adamou N, Umar UA. Effectiveness of preoperative tranexamic acid in reducing blood loss during caesarean section at Aminu Kano teaching Hospital, Kano: a randomized controlled trial. Pan Afr Med J 2021; 39: 34. https://doi.org/10.11604/pamj.2021.39.34.21938
Ray I, Bhattacharya R, Chakraborty S, Bagchi C, Mukhopadhyay S. Role of intravenous tranexamic acid on Caesarean blood loss: a prospective randomised study. J Obstet Gynecol India 2016; 66: 347–52. https://doi.org/10.1007/s13224-016-0915-x
Sanad, Ellakwa, Gomaa, Hamza, Elsalamony. Effect of tranexamic acid in reducing blood loss during and after Cesarean delivery. Menoufia Medical Journal 2020; 33:1270–5
Sentilhes L, Sénat MV, Le Lous M, et al. Tranexamic acid for the prevention of blood loss after Cesarean delivery. N Engl J Med 2021; 384: 1623–34. https://doi.org/10.1056/nejmoa2028788
Sentürk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for Cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet 2013; 287: 641–5. https://doi.org/10.1007/s00404-012-2624-8
Shabir N, Pirzada H, Hanif S, Rafique R. Tranexamic acid and blood loss during and after Cesarean section: a prospective randomized study. Int J Pathol 2019; 17: 190–5.
Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after Caesarean section. J Coll Physicians Surg Pak 2013; 23: 459–62.
Shalaby MA, Maged AM, Al-Asmar A, El Mahy M, Al-Mohamady M, Rund NM. Safety and efficacy of preoperative tranexamic acid in reducing intraoperative and postoperative blood loss in high-risk women undergoing Cesarean delivery: a randomized controlled trial. BMC Pregnancy Childbirth 2022; 22: 201. https://doi.org/10.1186/s12884-022-04530-4
Soliman AA, Mahmoud SA, Dawood RM, Fayed AA, Fathey AA. Prophylactic use of tranexamic acid in reducing blood loss during elective Cesarean section. Egypt J Hosp Med 2021; 82: 6–10. https://doi.org/10.21608/ejhm.2021.137140
Sujata N, Tobin R, Kaur R, Aneja A, Khanna M, Hanjoora VM. Randomized controlled trial of tranexamic acid among parturients at increased risk for postpartum hemorrhage undergoing Cesarean delivery. Int J Gynecol Obstet 2016; 133: 312–5. https://doi.org/10.1016/j.ijgo.2015.09.032
Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after Cesarean section: a double-blind randomization trial. Arch Gynecol Obstet 2013; 287: 463–8. https://doi.org/10.1007/s00404-012-2593-y
Yehia AH, Koleib MH, Abdelazim IA, Atik A. Tranexamic acid reduces blood loss during and after Cesarean section: a double blinded, randomized, controlled trial. Asian Pac J Reprod 2014; 3: 53–6. https://doi.org/10.1016/S2305-0500(14)60002-6
Howard DC, Jones AE, Skeith A, Lai J, D’Souza R, Caughey AB. Tranexamic acid for the treatment of postpartum hemorrhage: a cost-effectiveness analysis. Am J Obstet Gynecol MFM 2022; 4: 100588. https://doi.org/10.1016/j.ajogmf.2022.100588
Taeuber I, Weibel S, Herrmann E, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis, and meta-regression. JAMA Surg 2021; 156: e210884. https://doi.org/10.1001/jamasurg.2021.0884
Seifert SM, Lumbreras-Marquez MI, Goobie SM, et al. Tranexamic acid administered during Cesarean delivery in high-risk patients: maternal pharmacokinetics, pharmacodynamics, and coagulation status. Am J Obstet Gynecol 2022; 227: 763. https://doi.org/10.1016/j.ajog.2022.06.001
Author contributions
Henrique Provinciatto and Sara Amaral contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Maria E. Barbalho and Pedro M. da Câmara contributed to the data acquisition and interpretation of data. Isabelle B. Donadon, Luisa M. Fonseca, and Alice D. Marinho contributed to the acquisition of data. Eduardo Sirena contributed to the data analysis. Alexandre Provinciatto contributed to the design and interpretation of data.
Acknowledgments
The authors sincerely thank Dr. Rhanderson Cardoso (Brigham and Women’s Hospital, Harvard Medical School) for his review of the manuscript.
Disclosures
The authors declare no conflicts of interest.
Funding statement
The authors did not receive support from any organization for the submitted work.
Prior conference presentations
This meta-analysis was presented in the category of oral presentation at XXXVII Congresso Latino-americano de Anestesiologia (7–10 September 2023, Rio de Janeiro, Brazil).
Data availability statement
Because this meta-analysis was based on data extracted from previously published research, all the data and study materials are available in the public domain. The authors of this meta-analysis do not have access to patient-level data of the individual studies. Researchers interested in individual-level data from the studies included in this meta-analysis are encouraged to contact the corresponding author from each study with this request.
Editorial responsibility
This submission was handled by Dr. Ronald B. George, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Provinciatto, H., Barbalho, M.E., da Câmara, P.M. et al. Prophylactic tranexamic acid in Cesarean delivery: an updated meta-analysis with a trial sequential analysis. Can J Anesth/J Can Anesth 71, 465–478 (2024). https://doi.org/10.1007/s12630-024-02715-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-024-02715-3