Abstract
The phloem sap-sucking hemipteran insect, Aphis craccivora, commonly known as cowpea aphid, cause major yield loss of important food legume crop chickpea. Among different plant lectins Allium sativum leaf agglutinin (ASAL), a mannose binding lectin was found to be potent antifeedant for sap sucking insect A. craccivora. Present study describes expression of ASAL in chickpea through Agrobacterium-mediated transformation of “single cotyledon with half embryo” explant. ASAL was expressed under the control of CaMV35S promoter for constitutive expression and phloem specific rolC promoter for specifically targeting the toxin at feeding site, using pCAMBIA2301 vector containing plant selection marker nptII. Southern blot analysis demonstrated the integration and copy number of chimeric ASAL gene in chickpea and its inheritance in T1 and T2 progeny plants. Expression of ASAL in T0 and T1 plants was confirmed through northern and western blot analysis. The segregation pattern of ASAL transgene was observed in T1 progenies, which followed the 3:1 Mendelian ratio. Enzyme linked immunosorbant assay (ELISA) determined the level of ASAL expression in different transgenic lines in the range of 0.08–0.38% of total soluble protein. The phloem tissue specific expression of ASAL gene driven by rolC promoter has been monitored by immunolocalization analysis of mature stem sections. Survival and fecundity of A. craccivora decreased to 11–26% and 22–42%, respectively when in planta bioassay conducted on T1 plants compared to untransformed control plant which showed 85% survival. Thus, through unique approach of phloem specific expression of novel insecticidal lectin (ASAL), aphid resistance has been successfully achieved in chickpea.





Similar content being viewed by others
References
Babaoglu M, Davey MR, Power JB (2000) Genetic engineering of grain legumes: key transformation events. AgBiotechNet 2:1–12
Bandyopadhyay S, Roy A, Das S (2001) Binding of garlic (Allium sativum) leaf lectin to the gut receptors of homopteran pests is correlated to its insecticidal activity. Plant Sci 161:1025–1033. doi:10.1016/S0168-9452(01)00507-6
Banerjee S, Hess D, Majumder P, Roy D, Das S (2004) The interactions of Allium sativum leaf agglutinin with a chaperonin group of unique receptor protein isolated from a bacterial endosymbiont of the mustard aphid. J Biol Chem 279:23782–23789. doi:10.1074/jbc.M401405200
Bradford MM (1976) A rapid and sensitive method for the quantitation of proteins using the principle of protein–dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Chakraborti D, Sarkar A, Gupta S, Das S (2006a) Small and large scale genomic DNA isolation protocol for chickpea (Cicer arietinum L.), suitable for molecular marker and transgenic analyses. Afr J Biotechnol 5:585–589
Chakraborti D, Sarkar A, Das S (2006b) Efficient and rapid in vitro plant regeneration system for Indian cultivars of chickpea (Cicer arietinum L.). Plant Cell Tissue Organ Cult 86:117–123. doi:10.1007/s11240-005-9072-0
Chakraborti D, Sarkar A, Mondal HA, Schuermann D, Hohn B, Sarmah BK, Das S (2008) Cre/lox system to develop selectable marker free transgenic tobacco plants conferring resistance against sap sucking homopteran insect. Plant Cell Rep 27:1623–1633. doi:10.1007/s00299-008-0585-y
Chi H (1997) Computer program for the Probit Analysis. National Chung Hsing University, Taichung, Taiwan
Christou P, McCabe DE (1992) Prediction of germ-line transformation events in chimeric R0 transgenic soybean plantlets using tissue-specific expression patterns. Plant J 2:283–290. doi:10.1111/j.1365-313X.1992.00283.x
Dutta I, Saha P, Majumder P, Sarkar A, Chakraborti D, Banerjee S, Das S (2005a) The efficacy of a novel insecticidal protein, Allium sativum leaf lectin (ASAL), against homopteran insects monitored in transgenic tobacco. Plant Biotechnol J 3:601–611. doi:10.1111/j.1467-7652.2005.00151.x
Dutta I, Majumder I, Saha P, Ray K, Das S (2005b) Constitutive and phloem specific expression of Allium sativum leaf agglutinin (ASAL) to engineer aphid (Lipaphis erysimi) resistance in transgenic Indian mustard (Brassica juncea). Plant Sci 169:996–1007. doi:10.1016/j.plantsci.2005.05.016
Fitches E, Wiles D, Douglas AE, Hinchliffe G, Audsley N, Gatehouse JA (2008) The insecticidal activity of recombinant garlic lectins towards aphids. Insect Biochem Mol Biol 38:905–915. doi:10.1016/j.ibmb.2008.07.002
Fontana GS, Santini L, Caretto S, Frugis G, Mariotti D (1993) Genetic transformation in the grain legume Cicer arietinum L. (chickpea). Plant Cell Rep 12:194–198. doi:10.1007/BF00237052
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. doi:10.1016/0014-4827(68)90403-5
Hilder VA, Powell KS, Gatehouse AMR, Gatehouse J, Gatehouse LN, Shi Y, Hamilton W, Merryweather A, Newell CA, Timans JC (1995) Expression of snowdrop lectin in transgenic tobacco plants results in added protection against aphids. Transgenic Res 4:18–25. doi:10.1007/BF01976497
Indurker S, Misra HS, Eapen S (2007) Genetic transformation of chickpea (Cicer arietinum L.) with insecticidal crystal protein gene using particle gun bombardment. Plant Cell Rep 26:755–763. doi:10.1007/s00299-006-0283-6
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kar S, Johnson TM, Nayak P, Sen SK (1996) Efficient transgenic plant regeneration through Agrobacterium-mediated transformation of Chickpea (Cicer arietinum L.). Plant Cell Rep 16:32–37. doi:10.1007/BF01275444
Kar S, Basu D, Das S, Ramkrishnan NA, Mukherjee P, Nayak P, Sen SK (1997) Expression of cry1A(c) gene of Bacillus thuringiensis in transgenic chickpea plants inhibits development of pod-borer (Heliothis armigera) larvae. Transgenic Res 6:177–185. doi:10.1023/A:1018433922766
Krishnamurthy KV, Suhasini K, Sagare AP, Meixner M, de Kathen A, Pickardt T, Schieder O (2000) Agrobacterium mediated transformation of chickpea (Cicer arietinum L.) embryo axes. Plant Cell Rep 19:235–240. doi:10.1007/s002990050005
Loc NT, Tinjuangjun P, Gatehouse AMR, Christou P, Gatehouse JA (2002) Linear transgene constructs lacking vector backbone sequences generate transgenic rice plants which accumulate higher levels of proteins conferring insect resistance. Mol Breed 9:231–244. doi:10.1023/A:1020333210563
Majumder P, Banerjee S, Das S (2004) Identification of receptors responsible for binding of the mannose specific lectin to the gut epithelial membrane of the target insects. Glycoconj J 20:525–530. doi:10.1023/B:GLYC.0000043288.72051.7c
Majumder P, Mondal HA, Das S (2005) Insecticidal Activity of Arum maculatum tuber lectin and its binding to the glycosylated insect gut receptors. J Agric Food Chem 53:6727–6729. doi:10.1021/jf051155z
Matsuki R, Onodera H, Yamauchi T, Uchimiya H (1989) Tissue-specific expression of the rolC promoter of the Ri plasmid in transgenic rice plants. Mol Gen Genet 220:12–16. doi:10.1007/BF00260849
Matzke AJM, Matzke AM (1998) Position effect and epigenetic silencing of plant transgenes. Curr Opin Plant Biol 1:142–148. doi:10.1016/S1369-5266(98)80016-2
Murashige T, Skoog F (1962) A revised method for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Polowick PL, Baliski DS, Mahon JD (2004) Agrobacterium tumefaciens-mediated transformation of chickpea (Cicer arietinum L.): gene integration, expression and inheritance. Plant Cell Rep 23:485–491. doi:10.1007/s00299-004-0857-0
Powell KS (2001) Antifeedant effects of plant lectins towards nymphal stages of the planthoppers Tarophagous proserpina and Nilaparvata lugens. Entomol Exp Appl 99:71–77. doi:10.1023/A:1018948228640
Powell KS, Gatehouse AMR, Hilder VA, Gatehouse AJ (1995) Antifeedant effects of plant lectins and an enzyme on the adult stage of the rice brown planthopper, Nilaparvata lugens. Entomol Exp Appl 75:51–69. doi:10.1007/BF02382779
Ramesh S, Nagadhara D, Reddy VD, Rao KV (2004) Production of transgenic indica rice resistant to yellow stem borer and sap-sucking insects, using super-binary vectors of Agrobacterium tumefaciens. Plant Sci 166:1077–1085. doi:10.1016/j.plantsci.2003.12.028
Rao KV, Rathore KS, Hodges TK, Fu X, Stoger E, Sudhakar S, Williams P, Christou P, Bharathi M, Bown DP, Powell KS, Spence J, Gatehouse A, Gatehouse JA (1998) Expression of snowdrop lectin (GNA) in transgenic plants confers resistance to Rice Brown plant Hopper. Plant J 15:469–477. doi:10.1046/j.1365-313X.1998.00226.x
Reddy SV, Kumar PL (2004) Transmission and properties of a new leutovirus associated with chickpea stunt disease in India. Curr Sci 86:1157–1161
Roy A, Banerjee S, Majumder P, Das S (2002) Efficiency of mannose-binding plant lectins in controlling a homopteran insect, the red cotton bug. J Agric Food Chem 50:6775–6779. doi:10.1021/jf025660x
Roy A, Chakraborti D, Das S (2008) Effectiveness of garlic lectin on red spider mite of tea. J Plant Interact 3:157–162. doi:10.1080/17429140701754195
Sadeghi A, Broeders S, De Greve H, Hernalsteens J-P, Peumans WJ, Van Damme EJM, Smagghe G (2007) Expression of garlic leaf lectin under the control of the phloem-specific promoter Asus1 from Arabidopsis thaliana protects tobacco plants against the tobacco aphid (Myzus nicotianae). Pest Manag Sci 63:1215–1223. doi:10.1002/ps.1455
Sadeghi A, Smagghe G, Broeders S, Hernalsteens J-P, De Greve H, Peumans WJ, Van Damme EJM (2008) Ectopically expressed leaf and bulb lectins from garlic (Allium sativum L.) protect transgenic tobacco plants against cotton leafworm (Spodoptera littoralis). Transgenic Res 17:9–18. doi:10.1007/s11248-007-9069-z
Saha P, Majumder P, Dutta I, Ray T, Roy SC, Das S (2006) Transgenic rice expressing Allium sativum leaf lectin with enhanced resistance against sap-sucking insect pests. Planta 223:1329–1343. doi:10.1007/s00425-005-0182-z
Saha P, Chakraborti D, Sarkar A, Dutta I, Basu D, Das S (2007) Characterization of vascular specific RSs1 and rolC promoters for their utilization in engineering plants to develop resistance against hemipteran insect pests. Planta 226:429–442. doi:10.1007/s00425-007-0493-3
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sanyal I, Singh AK, Kaushik M, Amla DV (2005) Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci 168:1135–1146. doi:10.1016/j.plantsci.2004.12.015
Sarmah BK, Moore A, Tate W, Molvig L, Morton RL, Rees DP, Chiaiese P, Chrispeels MJ, Tabe LM, Higgins TJV (2004) Transgenic chickpea seeds expressing high levels of a bean α-amylase inhibitor. Mol Breed 14:73–82. doi:10.1023/B:MOLB.0000037996.01494.12
Sauvion N, Rahbé Y, Peumans WJ, Van Damme EJM, Gatehouse JA, Gatehouse AMR (1996) Effects of GNA and other mannose binding lectins on development and fecundity of the peach-potato aphid Myzus persicae. Entomol Exp Appl 79:285–293. doi:10.1007/BF00186287
Schmulling T, Schell J, Spena A (1989) Promoters of the rolA, B, and C genes of Agrobacterium rhizogenes are differentially regulated in transgenic plants. Plant Cell 1:665–670
Schuler TM, Poppy GM, Kerry BR, Denholm I (1998) Insect resistant transgenic plants. Trends Biotechnol 16:168–174. doi:10.1016/S0167-7799(97)01171-2
Senthil G, Williamson B, Dinkins RD, Ramsay G (2004) An efficient transformation system for chickpea (Cicer arietinum L.). Plant Cell Rep 23:297–303. doi:10.1007/s00299-004-0854-3
Sharma HC, Sharma KK, Crouch JH (2004) Genetic transformation of crops for insect resistance: potential and limitations. Crit Rev Plant Sci 23:47–72. doi:10.1080/07352680490273400
Somers DA, Samac DA, Olhoft PM (2003) Recent advances in legume transformation. Plant Physiol 131:892–899. doi:10.1104/pp.102.017681
Sonia, Singh RP, Sharma KK, Jaiwal PK (2003) In vitro regeneration and genetic transformation of chickpea. In: Jaiwal PK, Singh RP (eds) Applied genetics of leguminosae biotechnology. Kluwer Academic Publishers, Great Britain, pp 69–87
Sugaya S, Hayakawa K, Handa K, Uchimiya H (1989) Cell-specific expression of the rolC gene of the TL-DNA of Ri plasmid in transgenic tobacco plants. Plant Cell Physiol 30:649–653
Travella S, Ross SM, Harden J, Everett C, Snape JW, Harwood WA (2004) A comparison of transgenic barley lines produced by particle bombardment and Agrobacterium-mediated techniques. Plant Cell Rep 23:780–789. doi:10.1007/s00299-004-0892-x
Van Rheneen HA, Pundir RPS, Miranda JH (1993) How to accelerate the genetic improvement of a recalcitrant crop species such as chickpea. Curr Sci 654:414–417
Watanabe T, Kitagawa H (2000) Photosynthesis and translocation of assimilates in rice plants following phloem feeding by the plant hopper Nilaparvata lugens (Homoptera: Delphacidae). J Econ Entomol 93:1192–1198
Acknowledgements
We are grateful to Bose institute for providing infrastructure and other facilities to undergo the study. The authors are thankful to Swiss Agency for Development and Cooperation, Government of Switzerland and the Department of Biotechnology, Government of India under the Indo-Swiss Collaboration in Biotechnology for financial contributions. The help of Dr. K. K. Sharma, ICRISAT, Dr. B. K. Sarmah, AAU, Prof. Thomas Hohn and Prof. Barbara Hohn, Basel, Switzerland are sincerely acknowledged. Technical help from Mr. Arup Dey is being acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11248_2009_9242_MOESM2_ESM.jpeg
Artificial diet bioassay of (a) ASAL, (b) DEA, (c) CEA and (d) ATL against A. craccivora. Graphs showing mean survival per replicates, and each bar represents mean ± SE (JPEG 651 kb)
11248_2009_9242_MOESM3_ESM.jpeg
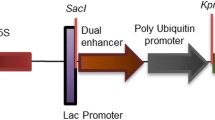
Schematic representation of the T-DNA region of binary vector constructs (a) pCAMBIA35SASAL and (b) pCAMBIArolCASAL showing the restriction sites. ASAL, gusA and neomycin phosphotransferase (nptII) coding genes are shown within T-DNA. LB, left border of T-DNA; RB, right border of T-DNA; CaMV35S Pr., cauliflower mosaic virus 35S promoter; rolC Pr., Agrobacterium rhizogenes rolC gene promoter; CaMV35SpolyA, cauliflower mosaic virus 35S terminator; nos polyA, nopaline synthase polyA terminator (JPEG 448 kb)
11248_2009_9242_MOESM4_ESM.jpeg
PCR analyses for segregation of ASAL gene in randomly chosen T1 progenies. (a) Lane 1, GenerulerTM (MBI Fermentus) marker; lane 2, pCAMBIA35S ASAL plasmid as positive control; lane 3, untransformed DNA as negative control; lanes 4–17, DNA samples of fourteen randomly selected T1 progenies of 35SASAL line cp212. Lanes 8, 10 and 13 did not show any amplification. (b) Lane 1, GenerulerTM (MBI Fermentus) marker; lane 2, pCAMBIArolCASAL plasmid as positive control; lane 3, untransformed DNA as negative control; lanes 4–18, DNA samples of fifteen randomly selected T1 progenies of rolCASAL line cp101. Lanes 10, 14, 17 and 18 did not show any amplification (JPEG 1769 kb)
Rights and permissions
About this article
Cite this article
Chakraborti, D., Sarkar, A., Mondal, H.A. et al. Tissue specific expression of potent insecticidal, Allium sativum leaf agglutinin (ASAL) in important pulse crop, chickpea (Cicer arietinum L.) to resist the phloem feeding Aphis craccivora . Transgenic Res 18, 529–544 (2009). https://doi.org/10.1007/s11248-009-9242-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-009-9242-7