Abstract
This paper deals with the ethanol (EtOH) removal in both dry and humid air fed dielectric barrier discharges. The experimental results were compared to the predictions of a zero dimension kinetic model to elucidate the main chemical routes occurring in the plasma phase. This comparison shows that both the dissociative quenching of the nitrogen metastables and the oxidation reactions by the oxygen atom or the hydroxyl radical should be taken into account to explain the EtOH abatement in these kinds of discharges. The CH3CHOH radical seems to be the main product of the nitrogen dissociative collisions, whereas radicals issued from the α- and β-H atom cleavage are the dominant ethanol oxidation by-products. These radicals account for the production of acetaldehyde, the main by-product of the ethanol/air fed discharges investigated here. Apart the complete oxidation products, i.e. carbon oxides and water, aldehydes containing up to six carbon atoms, ketones, carboxylic acids, ozone, nitrogen oxides, nitric acid and organic nitrates were found in the exhaust gas. A kinetic pathway is proposed to explain the formation of the detected by-products. Water vapour addition to the feeding gas slightly improves the EtOH removal and promotes further oxidation of the main by-products, thus enhancing the CO2 selectivity. This behaviour could be ascribed to the higher amount of hydroxyl radicals, which could boost the production of the direct precursors of CO2.







Similar content being viewed by others
References
Vandenbroucke AM, Morent R, De Geyter N, Leys C (2011) J Hazard Mater 195:30–54
Chang JS (2008) Plasma Sources Sci Technol 17:045004
Best Available Techniques Reference Documents (BREFs) in the framework of Article 13(1) of the Industrial Emissions Directive (IED). http://eippcb.jrc.ec.europa.eu/reference/
Nimlos MR, Wolfrum EJ, Brewer ML, Fennell JA, Bintner G (1996) Environ Sci Technol 30:3102–3110
Muggli DS, McCue JT, Falconer JL (1998) J Catal 173:470–483
Kovalenko VV, Rumyantseva MN, Gaskov AM, Makshina EV, Yushchenko VV, Ivanova II, Ponzoni A, Faglia G, Comini E (2006) Inorg Mater 42:1088–1093
Y-P I, Liu Y-C, Han K-Y, She T-C (2004) Environ Sci Technol 38:3785–3791
Lyulyukin MN, Besov AS, Vorontsov AV (2013) Ind Eng Chem Res 52:5842–5848
Aubry O, Met C, Khacef A, Cormier JM (2005) Chem Eng J 106:241–247
Wang B, Lü Y, Zhang X, Hu S (2011) J Nat Gas Chem 20:151–154
Wang W, Zhu C, Cao Y (2010) Int J Hydrog Energy 35:1951–1956
Marinov NM (1999) Int J Chem Kinet 31:183–220
Li J, Kazakov A, Dryer FL (2004) J Phys Chem A 108:7671–7680
Gupta GK, Dean AM, Ahn K, Gorte RJ (2006) J Power Sources 158:497–503
Esarte C, Millera A, Bilbao R, Alzueta MU (2009) Fuel Process Technol 90:496–503
Li A, Zhang S, Reznik B, Lichtenberg S, Schoch G, Deutschmann O (2011) Proc Combust Inst 33:1843–1850
Méricam-Bourdet N, Kirkpatrick M, Frochot D, Odic E, Tuvache F (2011) ecleer, Energy efficiency for industry. http://www.ecleer.fr/web/guest/industry/publications
Méricam-Bourdet N (2012) PhD thesis, Supélec Engineering School, France
Frochot D, Tuvache F (2012) European Patent EP2120514B1
Lovascio S, Blin-Simiand N, Jorand F, Jeanney P, Pasquiers S (2012) Int J Plasma Environ Sci Technol 6:111–118
Blin-Simiand N, Pasquiers S, Jorand F, Magne L, Postel C (2010) In: International symposium on non-thermal/thermal plasma pollution control technology and sustainable energy (ISNTP-7) St. John’s, Terre Neuve, Canada. Proceedings in CD-ROM
Méricam-Bourdet N, Kirkpatrick MJ, Tuvache F, Frochot D, Odic E (2012) Eur Phys J Appl Phys 57:30801
Méricam-Bourdet N, Kirkpatrick MJ, Tuvache F, Odic E, Frochot D (2011) Proceedings of the 30th international conference on phenomena in ionized gases (ICPIG), Belfast, Northern Ireland, UK, Paper D14–214
Korolevich M, Sivchik V, Zhbankov R, Lastochkina V (1986) J Appl Spectrosc 45:1275
Allen G, Remedios J, Newnham D, Smith K, Monks P (2004) Atmos Chem Phys Discuss 4:5655
Blin-Simiand N, Jorand F, Magne L, Pasquiers S, Postel C, Vacher J-R (2008) Plasma Chem Plasma Process 28:429–466
Mfopara A, Kirkpatrick MJ, Odic E (2009) Plasma Chem Plasma Process 29:91–102
Bo Zh, Yan JH, Li XD, Chi Y, Cen KF, Chéron BG (2007) Plasma Chem Plasma Process 27:546–558
Falkenstein Z, Coogan JJ (1997) J Phys D Appl Phys 30:817–825
Rosocha LA, Korzekwa RA (1999) J Adv Oxid Technol 4:247
Yan K, van Heesch EJM, Pemen AJM, Huijbrechts PAHJ (2001) Plasma Chem Plasma Process 21:107–137
Koeta O, Pasquiers S, Blin-Simiand N, Jorand F, Bary A (2012) ESCAMPIG XXI, Viana do Castelo, Portugal. Proceedings, paper P2.1.20
Koeta O, Pasquiers S, Blin-Simiand N, Jorand F, Bary A (2012) Int J Plasma Environ Sci Technol 6:227–232
NIST Chemical Kinetics Database, Standard Reference Database 17, Version 7.0 (Web Version), Release 1.6.5, Data Version 2012.02. http://kinetics.nist.gov/kinetics/
Blin-Simiand N, Pasquiers S, Jorand F, Postel C, Vacher J-R (2009) J Phys D Appl Phys 42:122003
Faider W, Pasquiers S, Blin-Simiand N, Magne L (2013) Plasma Sources Sci Technol 22:065010
Pasquiers S, Blin-Simiand N, Magne L (2013) Plasma Phys Control Fusion 55:124023
Frish M et al (2004) Gaussian 03, Revision C.02. Gaussian Inc., Wallingford
Faider W, Pasquiers S, Blin-Simiand N, Magne L (2013) J Phys D Appl Phys 46:105202
Pasquiers S, Faider W, Blin-Simiand N, Magne L, Jeanney P, Jorand F (2012) Int J Plasma Environ Sci Technol 6:149–155
Teodoru S, Kusano Y, Bogaerts A (2012) Plasma Process Polym 9:652–689
Hoard J, Wallington TJ, Bretz RL, Malkin A, Dorai R, Kushner MJ (2003) Int J Chem Kinet 35:231–238
Rudolph R, Francke K-P, Miessner H (2003) Plasma Polym 8:153–161
Yu-fang G, Dai-qi Y, Ke-fu C, Ya-feng T (2006) Plasma Chem Plasma Process 26:237–249
Acknowledgments
The authors thank the French Agency for Research (Agence Nationale de la Recherche) for its support of the PECCOVAIR Project (Grant ANR-09-ECOT-013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11090_2014_9601_MOESM2_ESM.tif
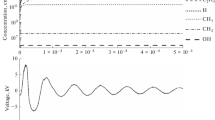
Ozone concentration in dry air DBDs (air flow: 2l/min NTP). For the higher and lower value limits see the “Experimental” and “Modeling” sections in the text and reference [26]. (TIFF 2,899 kb)
Rights and permissions
About this article
Cite this article
Lovascio, S., Blin-Simiand, N., Magne, L. et al. Experimental Study and Kinetic Modeling for Ethanol Treatment by Air Dielectric Barrier Discharges. Plasma Chem Plasma Process 35, 279–301 (2015). https://doi.org/10.1007/s11090-014-9601-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-014-9601-x