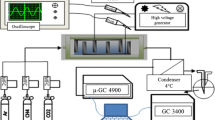
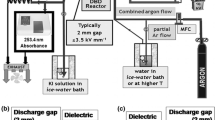
Abstract
The effect of the volumetric flow rate of reaction mixture components on the nonoxidative reforming of methane with admixed water in dielectric-barrier discharge has been studied. An increase in the volumetric flow rate of water from 1.3 to 6 cm3/h did not exert a noticeable effect on the conversion of methane and on the concentrations of hydrogen and ethane in gaseous reaction products. An increase in the concentration of propane and butanes was observed, whereas the total ethylene and propylene content of the products decreased. An increase in the volumetric flow rate of methane from 5 to 60 cm3/min led to a decrease in the conversion of methane from 29.3 to 6.2 vol % and a decrease in energy consumption for its reforming from 88.3 to 34.7 eV/molecule, and it was also accompanied by a decrease in the rate of formation of gaseous alkanes and olefins. The experimental data confirmed previous conclusions on the reaction mechanism and kinetics of the nonoxidative reforming of methane in dielectric-barrier discharge, which were made using a mathematical model.




Similar content being viewed by others
REFERENCES
Kudryashov, S.V., Ryabov, A.Y., and Ochered’ko, A.N., High Energy Chem., 2017, vol. 51, no. 2, p. 128.
Scapinello, M., Delikonstantis, E., and Stefanidis, G.D., Fuel, 2018, vol. 222, p. 705.
Michiels, D., Leys, Ch., Geem, K.M.V., Marin, G.B., and Nikiforov, A., J. Clean Prod., 2019, vol. 209, p. 655.
Jeon, B., Park, E.D., and Kim, YuK., Res. Chem. Intermed., 2018, vol. 44, no. 6, p. 3761.
Khoja, A.H., Tahir, M., and Amin, N.A.S., Energy Convers. Manage., 2019, vol. 183, p. 529.
Moshrefi, M.M. and Rashidi, F., Plasma Chem. Plasma Process., 2018, vol. 38, no. 3, p. 503.
Khadir, N., Khodja, K., and Belasri, A., Plasma Sci. Technol., 2017, vol. 19, no. 9, p. 095502.
Lim, M.S. and Chun, Y.N., Plasma Chem. Plasma Process., 2016, vol. 36, no. 5, p. 1211.
Indarto, A., Coowanitwong, N., Choi, J.-W., Lee, H., and Song, H.K., Fuel Process. Technol., 2008, vol. 89, no. 2, p. 214.
Shapoval, V. and Marotta, E., Plasma Process. Polym., 2015, vol. 12, no. 8, p. 808.
Kadoa, S., Sekine, Y., Nozaki, T., and Okazaki, K., Catal. Today, 2004, vol. 89, nos. 1–2, p. 47.
Dors, M., Nowakowska, H., Jasinski, M., and Mizeraczyk, J., Plasma Chem. Plasma Process., 2014, vol. 34, no. 2, p. 313.
Kudryashov, S.V., Ryabov, A.Y., and Ochered’ko, A.N., High Energy Chem., 2018, vol. 52, no. 2, p. 167.
Luque, J., Database and Spectral simulation for diatomic molecules. Lifebase 2.0. http://www.sri.com/psd/lifbase. Accessed March 23, 2019.
Zabarnick, S., Fleming, J.W., and Lin, M.C., Symp. Combust., 1988, vol. 21, no. 1, p. 713.
Braun, W., McNesby, J.R., and Bass, A.M., J. Chem. Phys., 1967, vol. 46, p. 2071.
Pushkarev, A.I., Zhu, A.-M., Li, X.-S., and Sazonov, R.V., High Energy Chem., 2009, vol. 43, no. 3, p. 156.
Funding
This work was carried out in accordance with the Program of Fundamental Scientific Research of State Academies of Sciences for 2013–2020, project no. V.44.3.1 (state registration no. AAAA-A17-117030310198-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Ryabov, A.Y., Kudryashov, S.V. & Ochered’ko, A.N. Effect of the Volumetric Flow Rate of Reaction Mixture Components on Nonoxidative Reforming of Methane with Admixed Water in Dielectric-Barrier Discharge. High Energy Chem 53, 478–481 (2019). https://doi.org/10.1134/S0018143919060134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143919060134