Abstract
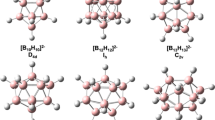
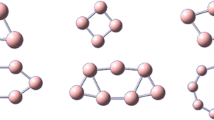
The double-chain boron ribbon should be taken as an element geometric unit for the construction of boron fullerenes and sheets. In this work, a series of B2n C2H2 clusters (n = 2–9) were extensively investigated using the density functional theory and the coupled cluster method. The most stable structures of B2n C2H2 are planar double-chain nanoribbon with lengths from 3.9 to 15.0 Å. The adaptive natural density partitioning analyses show that there exist conjugated multi-center π bonds along the nanoribbons. The two-dimensional contour pictures of the nucleus-independent chemical shifts reveal that B2n C2H2 clusters have ribbon aromaticity that fluctuates along the ribbons. This finding will provide new insights on the boron fullerenes and the two-dimensional boron sheets.






Similar content being viewed by others
References
A. N. Alexandrova, A. I. Boldyrev, H. J. Zhai, and L. S. Wang (2006). Coord. Chem. Rev. 250, 2811.
I. Boustani (1997). Phys. Rev. B. 55, 16426.
J. E. Fowler and J. M. Ugalde (2000). J. Phys. Chem. A. 104, 397.
H. J. Zhai, A. N. Alexandrova, K. A. Birch, A. I. Boldyrev, and L. S. Wang (2003). Angew. Chem. Int. Ed. 42, 6004.
H. J. Zhai, B. Kiran, J. Li, and L. S. Wang (2003). Nat. Mater. 2, 827.
J. I. Aihara, H. Kanno, and T. Ishida (2005). J. Am. Chem. Soc. 127, 13324.
B. Kiran, S. Bulusu, H. J. Zhai, S. Yoo, X. C. Zeng, and L. S. Wang (2005). Proc. Natl. Acad. Sci. USA 102, 961.
D. Yu. Zubarev and A. I. Boldyrev (2007). J. Comput. Chem. 28, 251.
E. Oger, N. R. M. Crawford, R. Kelting, P. Weis, M. M. Kappes, and R. Ahlrichs (2007). Angew. Chem. Int. Ed. 46, 8503.
A. P. Sergeeva, D. Yu. Zubarev, H. J. Zhai, A. I. Boldyrev, and L. S. Wang (2008). J. Am. Chem. Soc. 130, 7244.
W. Huang, A. P. Sergeeva, H. J. Zhai, B. B. Averkiev, L. S. Wang, and A. I. Boldyrev (2010). Nat. Chem. 2, 202.
A. P. Sergeeva, B. B. Averkiev, H. J. Zhai, A. I. Boldyrev, and L. S. Wang (2011). J. Chem. Phys. 134, 224304.
A. P. Sergeeva, Z. A. Piazza, C. Romanescu, W. L. Li, A. I. Boldyrev, and L. S. Wang (2012). J. Am. Chem. Soc. 134, 18065.
A. P. Sergeeva, I. A. Popov, Z. A. Piazza, W. L. Li, C. Romanescu, L. S. Wang, and A. I. Boldyrev (2014). Acc. Chem. Res. 47, 1349.
Z. A. Piazza, I. A. Popov, W. L. Li, R. Pal, X. C. Zeng, A. I. Boldyrev, and L. S. Wang (2014). J. Chem. Phys. 141, 034303.
W. L. Li, Y. F. Zhao, H. S. Hu, J. Li, and L. S. Wang (2014). Angew. Chem. Int. Ed. 126, 5646.
W. L. Li, Q. Chen, W. J. Tian, H. Bai, Y. F. Zhao, H. S. Hu, J. Li, H. J. Zhai, S. D. Li, and L. S. Wang (2014). J. Am. Chem. Soc. 136, 12257.
Q. Chen, G. F. Wei, W. J. Tian, H. Bai, Z. P. Liu, H. J. Zhai, and S. D. Li (2014). Phys. Chem. Chem. Phys. 16, 18282.
H. Bai, Q. Chen, H. J. Zhai, and S. D. Li (2015). Angew. Chem. Int. Ed. 54, 941.
J. K. Olson and A. I. Boldyrev (2013). J. Phys. Chem. A. 117, 1614.
A. N. Alexandrova, E. Koyle, and A. I. Boldyrev (2006). J. Mol. Model. 12, 569.
N. G. Szwacki, V. Weber, and C. J. Tymczak (2009). Nanoscale Res. Lett. 4, 1085.
Q. Chen, H. Bai, J. C. Guo, C. Q. Miao, and S. D. Li (2011). Phys. Chem. Chem. Phys. 13, 20620.
D. Z. Li, H. G. Lu, and S. D. Li (2012). J. Mol. Model. 18, 3161.
Q. Chen and S. D. Li (2011). J. Clust. Sci. 22, 513.
H. Bai, Q. Chen, Y. F. Zhao, Y. B. Wu, H. G. Lu, J. Li, and S. D. Li (2013). J. Mol. Model. 19, 1195.
W. J. Tian, H. Bai, H. G. Lu, Y. B. Wu, and S. D. Li (2013). J. Clust. Sci. 24, 1127.
D. Z. Li, Q. Chen, Y. B. Wu, H. G. Lu, and S. D. Li (2012). Phys. Chem. Chem. Phys. 14, 14769.
W. L. Li, C. Romanescu, T. Jian, and L. S. Wang (2012). J. Am. Chem. Soc. 134, 13228.
H. J. Zhai, Q. Chen, H. Bai, H. G. Lu, W. L. Li, S. D. Li, and L. S. Wang (2013). J. Chem. Phys. 139, 174301.
H. Bai, Q. Chen, C. Q. Miao, Y. W. Mu, Y. B. Wu, H. G. Lu, H. J. Zhai, and S. D. Li (2013). Phys. Chem. Chem. Phys. 15, 18872.
H. J. Zhai, Y. F. Zhao, W. L. Li, Q. Chen, H. Bai, H. S. Hu, Z. A. Piazza, W. J. Tian, H. G. Lu, Y. B. Wu, Y. W. Mu, G. F. Wei, Z. P. Liu, J. Li, S. D. Li, and L. S. Wang (2014). Nat. Chem. 6, 727.
Q. Chen, W. L. Li, Y. F. Zhao, S. Y. Zhang, H. S. Hu, H. Bai, H. R. Li, W. J. Tian, H. G. Lu, H. J. Zhai, S. D. Li, J. Li, and L. S. Wang (2015). ACS Nano. 9, 754.
L. Wang, J. Zhao, F. Li, and Z. Chen (2010). Chem. Phys. Lett. 501, 16.
F. Li, P. Jin, D. Jiang, L. Wang, S. B. Zhang, J. Zhao, and Z. Chen (2013). J. Chem. Phys. 136, 074302.
S. Polad and M. Ozaybcd (2013). Phys. Chem. Chem. Phys. 15, 19819.
J. Lv, Y. Wang, L. Zhu, and Y. Ma (2014). Nanoscale 6, 11692.
H. Lu and S.-D. Li (2013). J. Chem. Phys. 139, 2243071.
H. Tang and S. Ismail-Beigi (2007). Phys. Rev. Lett. 99, 115501.
X. Yang, Y. Ding, and J. Ni (2008). Phys. Rev. B 77, 041402.
X. Wu, J. Dai, Y. Zhao, Z. Zhuo, J. Yang, and X. C. Zeng (2012). ACS Nano 6, 7443.
E. S. Penev, S. Bhowmick, A. Sadrzadeh, and B. I. Yakobson (2012). Nano Lett. 12, 2441.
H. G. Lu, Y. W. Mu, H. Bai, Q. Chen, and S. D. Li (2013). J. Chem. Phys. 138, 024701.
Y. Liu, E. S. Penev, and B. I. Yakobson (2013). Angew. Chem. Int. Ed. 52, 3156.
H. Liu, J. Gao, and J. Zhao (2013). Sci. Rep. 3, 3238.
H. Bai and S.-D. Li (2011). J. Clust. Sci. 22, 525.
P. V. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, and N. J. R. V. E. Hommes (1996). J. Am. Chem. Soc. 118, 6317.
Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta, and P. V. R. Schleyer (2005). Chem. Rev. 105, 3842–3888.
D. Yu. Zubarev and A. I. Boldyrev (2008). Phys. Chem. Chem. Phys. 10, 5207.
I. A. Popov, K. V. Bozhenko, and A. I. Boldyrev (2012). Nano Res. 5, 117–123.
R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople (1980). J. Chem. Phys. 72, 650.
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B. 37, 785.
J. Cizek (1969). Adv. Chem. Phys. 14, 35.
G. E. Scuseria and H. F. Schaefer (1989). J. Chem. Phys. 90, 3700.
R. J. Bartlett and M. Musial (2007). Rev. Mod. Phys. 79, 291.
M. E. Casida, C. Jamorski, K. C. Casida, and D. R. Salahub (1998). J. Chem. Phys. 108, 4439.
R. Bauernschmitt and R. Ahlrichs (1996). Chem. Phys. Lett. 256, 454.
K. Wolinski, J. F. Hilton, and P. Pulay (1990). J. Am. Chem. Soc. 112, 8251–8260.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, et al. Gaussian 09, D.01 (Gaussian, Inc., Wallingford, CT, 2009).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants Nos. 21373130 and 21473106). We also thank the High-Performance Computing Platform of Shanxi University for funding the computer time.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, SY., Bai, H., Chen, Q. et al. Ribbon Aromaticity of Double-Chain B2n C2H2 Clusters (n = 2–9): A First Principle Study. J Clust Sci 26, 2043–2050 (2015). https://doi.org/10.1007/s10876-015-0903-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0903-9