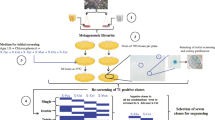
Abstract
Environments where lignocellulosic biomass is naturally decomposed are sources for discovery of new hydrolytic enzymes that can reduce the high cost of enzymatic cocktails for second-generation ethanol production. Metagenomic analysis was applied to discover genes coding carbohydrate-depleting enzymes from a microbial laboratory subculture using a mix of sugarcane bagasse and cow manure in the thermophilic composting phase. From a fosmid library, 182 clones had the ability to hydrolyse carbohydrate. Sequencing of 30 fosmids resulted in 12 contigs encoding 34 putative carbohydrate-active enzymes belonging to 17 glycosyl hydrolase (GH) families. One third of the putative proteins belong to the GH3 family, which includes β-glucosidase enzymes known to be important in the cellulose-deconstruction process but present with low activity in commercial enzyme preparations. Phylogenetic analysis of the amino acid sequences of seven selected proteins, including three β-glucosidases, showed low relatedness with protein sequences deposited in databases. These findings highlight microbial consortia obtained from a mixture of decomposing biomass residues, such as sugar cane bagasse and cow manure, as a rich resource of novel enzymes potentially useful in biotechnology for saccharification of lignocellulosic substrate.




Similar content being viewed by others
References
Altschul S, Gish W, Miller W, Myers E, Lipman D (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O (2008) The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi:10.1186/1471-2164-9-75
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270. doi:10.1016/0168-1656(92)90074-J
Balat M, Balat H (2009) Recent trends in global production and utilization of bioethanol fuel. Appl Energy 86:2273–2282. doi:10.1016/j.apenergy.2009.03.015
Belaich A, Parsiegla G, Gal L, Villard C, Haser R, Belaich JP (2002) Cel9 M, a new family 9 cellulase of the Clostridium cellulolyticum cellulosome. J Bacteriol 184:1378–1384. doi:10.1128/JB.184.5.1378-1384.2002
Beloqui A, Nechitaylo TY, López-Cortés N, Ghazi A, Guazzaroni ME, Polaina J, Strittmatter AW, Reva O, Waliczek A, Yakimov MM, Golyshina OV, Ferrer M, Golyshin PN (2010) Diversity of glycosyl hydrolases from cellulose depleting communities enriched from casts of two earthworm species. Appl Environ Microbiol 76:5934–5946. doi:10.1128/AEM.00902-10
Biver S, Stroobants A, Portetelle D, Vandenbol M (2014) Two promising alkaline β-glucosidases isolated by functional metagenomics from agricultural soil, including one showing high tolerance towards harsh detergents, oxidants and glucose. J. Ind Microbiol Biotechnol 41:479–488. doi:10.1007/s10295-014-1400-0
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Camargo PD (2005) Força verde: um novo campo para a indústria química. Revista Brasileira de Engenharia Química: 18–21
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238. doi:10.1093/nar/gkn663
Chen H, Li X, Ljundahl LG (1994) Isolation and properties of an extracellular beta-glucosidase from the polycentric rumen Fungus Orpinomyces sp. strain PC-2. Appl Environ Microbiol 60:64–70
Cowan D, Meyer Q, Stafford W, Muyanga S, Cameron R, Wittwer P (2005) Metagenomic gene discovery: past, present and future. Trends Biotech 23:321–329. doi:10.1016/j.tibtech.2005.04.001
de Oliveira MNV, Jewell KA, Freitas FS, Benjamin LA, Tótola MR, Borges AC, Moraes CA, Suen G (2013) Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet Microbiol 164:307–314. doi:10.1016/j.vetmic.2013.02.013
Del Pozo MV, Fernandéz-Arrojo L, Gil-Martínez J, Montesinos A, Chernikova TN, Nechitaylo TY, Waliszek A, Tortajada M, Rojas A, Huws SA, Golyshina OV, Newbold CJ, Polaina J, Ferre M, Golyshin PN (2012) Microbial β-glucosidases from cow rumen metagenome enhance the saccharification of lignocellulose in combination with commercial cellulase cocktail. Biotechnol Biofuels 5:1–13. doi:10.1186/1754-6834-5-73
Doi RH, Kosugi A (2004) Cellulosomes: plant-cell-wall-degrading enzyme complexes. Nat Rev Microbiol 2:541–551. doi:10.1038/nrmicro925
Duan CJ, Feng JX (2010) Mining metagenomes for novel cellulase genes. Biotechnol Lett 32:1765–1775. doi:10.1007/s10529-010-0356-z
Duan CJ, Xian L, Zhao GC, Feng Y, Pang H, Bai XL, Tang JL, Ma QS, Feng JX (2009) Isolation and partial characterization of novel genes encoding acidic cellulases from metagenomes of buffalo rumens. J Appl Microbiol 107:245–256. doi:10.1111/j.1365-2672.2009.04202.x
Eberhart B, Cross DF, Chase LR (1964) Beta-glucosidase system of Neurospora crass. I. beta-glucosidase and cellulose activities of mutant and wild-type strains. J Bacteriol 87:761–770
Feng Y, Duan CJ, Pang H, Mo XC, Wu CF, Yt Y, Hu YL, Wi J, Tang JL, Feng JX (2009) Cloning and identification of novel cellulase genes from uncultured microorganisms in rabbit cecum and characterization of the expressed cellulases. Appl Microbiol Biotechnol 75:319–328. doi:10.1007/s00253-006-0820-9
Ferrer M, Ghazi A, Beloqui A, Vieites JM, López-Cortéz N, Marín-Navarro J, Necgutaylo TY, Guazzaroni ME, Polaina J, Waliczek A, Chernikova TN, Reva ON, Golyshina OV, Golyshin PN (2012) Functional metagenomics unveils a multifunctional glycosyl hydrolase from the family 43 catalysing the breakdown of plant polymers in the calf rumen. PLoS One 7:e38134. doi:10.1371/journal.pone.0038134
Ghose TK (1987) Measurement of cellulase activity. Pure Appl Chem 59:257–268
Gnansounou E, Dauriat A (2010) Techno-economic analysis of lignocellulosic ethanol: a review. Bioresour Technol 101:4980–4991. doi:10.1016/j.biortech.2010.02.009
Grant S, Sorokin DY, Grant WD, Jones BE, Heaphy S (2004) A phylogenetic analysis of Wadi el Natrun soda lake cellulase enrichment cultures and identification of cellulase genes from these cultures. Extremophiles 8:421–429. doi:10.1007/s00792-004-0402-7
Handelsman J (2004) Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev 68:669–685. doi:10.1128/MMBR.68.4.669-685.2004
Hess M, Sczyrba A, Egan R, Kim TW, Chokhawala H, Schroth G, Luo S, Clark DS, Chen F, Zhang T, Mackie RI, Pennacchio LA, Tringle SG, Visel A, Woyke T, Wang Z, Rubin EM (2011) Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 331:463–467. doi:10.1126/science.1200387
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–807. doi:10.1126/science.1137016
Hjort K, Bergstrom M, Adesina MF, Jansson JK, Smalla K, Sjöling S (2010) Chitinase genes revealed and compared in bacterial isolates, DNA extracts and a metagenomic library from a phytopathogen-suppressive soil. FEMS Microbiol Ecol 71:197–207. doi:10.1111/j.1574-6941.2009.00801.x
Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VGH (2012) Novel enzymes for the degradation of cellulose. Biotechnol Biofuels 5:45. doi:10.1186/1754-6834-5-45
Hu Y, Guimin Z, Aiying L, Jing C, Lixin M (2008) Cloning and enzymatic characterization of a xylanase gene from a soil-derived metagenomic library with an efficient approach. Appl Microbiol Biotechnol 80:823–830. doi:10.1007/s00253-008-1636-6
Huber R, Stetter KO (1992) The order Thermotogales. In: Rosenberg E (ed) The Prokaryotes. Springer, Berlin, pp 3809–3815
Jami E, Mizrahi I (2012) Composition and similarity of bovine rumen microbiota across individual animals. PLoS One 7:e33306. doi:10.1371/journal.pone.0033306
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kanokratana P, Eurwilaichitr L, Pootanakit K, Champreda V (2015) Identification of glycosyl hydrolases from a metagenomic library of microflora in sugarcane bagasse collection site and their cooperative action on cellulose degradation. J Biosci Bioeng 119:384–391. doi:10.1016/j.jbiosc.2014.09.010
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr Microbiol 57:503–507. doi:10.1007/s00284-008-9276-8
Kim YJ, Choi GS, Kim SB, Yoon GS, Kim YS, Ryu YW (2006) Screening and characterization of a novel esterase from a metagenomic library. Protein Expr Purif 45:315–323. doi:10.1016/j.pep.2005.06.008
Kim SJ, Lee CM, Kim MY, Yeo YS, Yoon SH, Kang HC, Koo BS (2007) Screening and characterization of an enzyme with beta-glucosidase activity from environmental DNA. J Microbiol Biotechnol 17:905–912
Kim SJ, Chang-Muk L, Bo-Ram H, Min-Yong K, Yun-Soo Y, Sang-Hong Y, Bon-Sung K, Hong-Ki J (2008) Characterization of a gene encoding cellulase from uncultured soil bacteria. FEMS Microbiol Lett 282:44–51. doi:10.1111/j.1574-6968.2008.01097.x
Larkin M, Blackshields G, Brown N, Chenna R, McGettigan P, McWillian H, Valentin F, Wallace I, Wilm A, Lopez R, Thompson J, Gibson T, Higgins D (2001) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi:10.1093/bioinformatics/btm404
Lee SW, Won K, Lim HK, Kim JC, Choi GJ, Cho KY (2004) Screening for novel lipolytic enzymes from uncultured soil microorganisms. Appl Microbiol Biotechnol 65:720–726. doi:10.1007/s00253-004-1722-3
Li LL, McrCorkle SR, Monchy S, Taghavi S, van der Lelie D (2009) Bioprospecting metagenomes: glycosyl hydrolases for converting biomass. Biotechnol Biofuels 2:10. doi:10.1186/1754-6834-2-1
Li R, Li L, Huang R, Sun Y, Mei X, Shen B, Shen Q (2014) Variations of culturable thermophilic microbe numbers and bacterial communities during the thermophilic phase of composting. World J Microbiol Biotechnol 30:1737–1746. doi:10.1007/s11274-013-1593-9
Lopes LD, Lima AOS, Taketani RG, Darias P, Silva LRF, Romagnoli EM, Louvandini H, Abdalla AL, Mendes R (2015) Exploring the sheep rumen microbiome for carbohydrate active enzymes. A Van Leeuwenhoek 118:15–30. doi:10.1007/s10482-015-0459-6
Lu HP, Wang YB, Huang SW, Lin CY, Wu M, Hsieh CH, Yu HT (2012) Metagenomic analysis reveals a functional signature for biomass degradation by cecal microbiota in the leaf-eating flying squirrel (Petaurista alborufus lena). BMC Genomics 13:466. doi:10.1186/1471-2164-13-466
Makonde HM, Boga HI, Osiemo Z, Mwirichia R, Mackenzie LC, Goker M, Klenk HS (2013) 16S-rRNA-based analysis of bacterial diversity in the gut of fungus-cultivating termites (Microtermes and Odontotermes species). A Van Leeuw 104:869–883. doi:10.1007/s10482-013-0001-7
Matsuzawa T, Kaneko S, Yaoi K (2015) Screening, identification, and characterization of a GH43 family β-xylosidase/α-arabinofuranosidase from a compost microbial metagenome. Appl Microbiol Biotechnol 99:8943–8954. doi:10.1007/s00253-015-6647-5
Meyer F, Paarmann D, Souza MD, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A, Wilkening J, Edwards RA (2008) The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinform 9:386. doi:10.1186/1471-2105-9-386
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anl Chem 31:426–428. doi:10.1021/ac60147a030
Montella S, Amore A, Faraco V (2015) Metagenomics for the development of new biocatalysts to advance lignocellulose saccharification for bioeconomic development. Crit Rev Biotechnol. doi:10.3109/07388551.2015.1083939
Nolling J, Breton G, Omelchenko MV, Makarova KS, Zeng Q, Gibson R, Lee HM, Dubois J, Qiu D, Hitti J, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR (2001) Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol 183:4823–4838. doi:10.1128/JB.183.16.4823-4838.2001
Omelchenko MV, Galperin MY, Wolf YI, Koonin EV (2010) Non-homologous isofunctional enzymes: a systematic analysis of alternative solutions in enzyme evolution. Biol Direct 5:31. doi:10.1186/1745-6150-5-31
Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, Crecy-Lagard V, Diaz N, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi N, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H, Olsen G, Olson R, Osterman A, Portnoy V, Pusch GD, Rodionov DA, Ruckert C, Steiner J, Stevens R, Thiele I, Vassieva O, Ye Y, Zagnitko O, Vonstein V (2005) The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res 33:5691–5702. doi:10.1093/nar/gki866
Pandey S, Gulat S, Goyal E, Sing S, Kumar K, Nain L, Saxena AK (2016) Construction and screening of metagenomic library derived from soil for β-1,4-endoglucanase gene. Biocatal Agric Biotechnol 5:186–192. doi:10.1016/j.bcab.2016.01.008
Pang H, Zhang P, Duan CJ, Mo VX, Tang JL, Feng JX (2009) Identification of cellulase genes from the metagenomes of compost soils and functional characterization of one novel endoglucanase. Curr Microbiol 58:404–408. doi:10.1007/s00284-008-9346-y
Parachin NS, Gorwa-Grauslund MF (2011) Isolation of xylose isomerases by sequence- and function-based screening from a soil metagenomic library. Biotechnol Biofuels 4:1–10. doi:10.1186/1754-6834-4-9
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31:20–31. doi:10.3109/07388551003757816
Rabelo SC, Fonseca NA, Andrade RR, Maciel Filho RR, Costa AC (2011) Ethanol production from enzymati/c hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenergy 35:2600–2607. doi:10.1016/j.biombioe.2011.02.042
Rattanachomsri U, Kanokratana P, Eurwilaichitr L, Igarashi Y, Champreda V (2011) Culture-independent phylogenetic analysis of the microbial community in industrial sugarcane bagasse feedstock piles. Biosci Biotechnol Biochem 75:232–239. doi:10.1271/bbb.100429
Souza RA (2012) Obtenção de Inoculante e de Coquetel Enzimático Lignocelulolítico a partir de Comunidades Microbianas Termofílicas. 2012. 56f. Dissertação (mestrado). Universidade Federal de Viçosa, Viçosa
Stevenson DM, Weimer PJ (2007) Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol 75:165–174. doi:10.1007/s00253-009-2033-5
Sukharnikov LO, Cantwell BJ, Podar M, Zhulin IB (2011) Cellulases ambiguous nonhomologous enzymes in a genomic perspective. Trends Biotechnol 29:473–479. doi:10.1016/j.tibtech.2011.04.008
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. doi:10.1016/S0960-8524(01)00212-7
Tamaru Y, Karita S, Ibrahim A, Chan H, Doi RH (2000) A large gene cluster for the Clostridium cellulovorans cellulosome. J Bacteriol 182:5906–5910. doi:10.1128/JB.182.20.5906-5910.200
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Teather RM, Wood PJ (1982) Use of Congo red polysaccharide interactions complex formation between Congo red and polysaccharide in detection and assay of polysaccharide hydrolases. Methods Enzymol 160:59–74
Uchiyama T, Miyazaki K (2009) Functional metagenomics for enzyme discovery: challenges to efficient screening. Curr Opin Biotechnol 20:616–622. doi:10.1016/j.copbio.2009.09.010
Warnecke F, Luginbuhl P, Ivanova N, Ghassemian M, Richardson TH et al (2007) Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 450:560–565. doi:10.1038/nature06269
Yun J, Kang S, Park S, Yoon H, Kim MJ, Heu S, Ryu S (2004) Characterization of a novel amylolytic enzyme encoded by a gene from a soil-derived metagenomic library. Appl Environ Microbiol 70:7229–7235. doi:10.1128/AEM.70.12.7229-7235.2004
Acknowledgments
This work was supported by CAPES, CNPq and FAPEMIG Brazilian funding agencies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Colombo, L.T., de Oliveira, M.N.V., Carneiro, D.G. et al. Applying functional metagenomics to search for novel lignocellulosic enzymes in a microbial consortium derived from a thermophilic composting phase of sugarcane bagasse and cow manure. Antonie van Leeuwenhoek 109, 1217–1233 (2016). https://doi.org/10.1007/s10482-016-0723-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0723-4