Abstract
Key message
MLO mediates pollen hydration.
Abstract
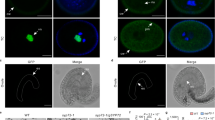
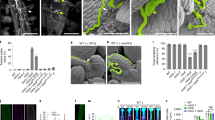
Hydration is the first step in pollen germination. However, the process is not well understood. OsMLO12 is highly expressed in mature pollen grains; plants containing alleles caused by transfer DNA insertions do not produce homozygous progeny. Reciprocal crosses between wild-type and OsMLO12/osmlo12 plants showed that the mutant alleles were not transmitted through the male gametophyte. Microscopic observations revealed that, although mutant grains became mature pollen with three nuclei, they did not germinate in vitro or in vivo due to a failure in hydration. The OsMLO12 protein has seven transmembrane motifs, with an N-terminal extracellular region and a C-terminal cytosolic region. We demonstrated that the C-terminal region mediates a calcium-dependent interaction with calmodulin. Our findings suggest that pollen hydration is regulated by MLO12, possibly through an interaction with calmodulin in the cytosol.






Similar content being viewed by others
References
Bahler M, Rhoads A (2002) Calmodulin signaling via the IQ motif. FEBS Lett 513:107–113
Bhat RA, Miklis M, Schmelzer E, Schulze-Lefert P, Panstruga R (2005) Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc Natl Acad Sci USA 102:3135–3140
Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Römheld V (2002) Boron in Plant Biology. Plant Biol 4:205–223
Buitink J, Claessens MM, Hemminga MA, Hoekstra FA (1998) Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Physiol 118:531–541
Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88:695–705
Cakmak I, Romheld V (1997) Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 193:71–83
Chen D, Ronald P (1999) A rapid DNA minipreparation method suitable for AFLP and other PCR applications. Plant Mol Biol Rep 17:53–57
Chen Z, Hartmann HA, Wu MJ, Friedman EJ, Chen JG, Pulley M, Schulze-Lefert P, Panstruga R, Jones AM (2006) Expression analysis of the AtMLO gene family encoding plant-specific seven-transmembrane domain proteins. Plant Mol Biol 60:583–597
Chen Z, Noir S, Kwaaitaal M, Hartmann HA, Wu MJ, Mudgil Y, Sukumar P, Muday G, Panstruga R, Jones AM (2009) Two seven-transmembrane domain MILDEW RESISTANCE LOCUS O proteins cofunction in Arabidopsis root thigmomorphogenesis. Plant Cell 21:1972–1991
Dannel F, Pfeffer H, Römheld V (2002) Update on boron in higher plants—uptake, primary translocation and compartmentation. Plant Biol 4:193–204
Devoto A, Piffanelli P, Nilsson I, Wallin E, Panstruga R, von Heijne G, Schulze-Lefert P (1999) Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J Biol Chem 274:34993–35004
Elleman CJ, Dickinson HG (1990) The role of the exine coating in pollen–stigma interactions in Brassica oleracea L. New Phytol 114:511–518
Elleman CJ, Franklin-Tong V, Dickinson HG (1992) Pollination in species with dry stigmas: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol 121:413–424
Fan LM, Wang YF, Wang H, Wu WH (2001) In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot 52:1603–1614
Fujita M, Horiuchi Y, Ueda Y, Mizuta Y, Kubo T, Yano K, Yamaki S, Tsuda K, Nagata T, Niihama M, Kato H, Kikuchi S, Hamada K, Mochizuki T, Ishimizu T, Iwai H, Tsutsumi N, Kurata N (2010) Rice expression atlas in reproductive development. Plant Cell Physiol 51:2060–2081
Hannoufa A, Mcnevin J, Lemieux B (1993) Epicuticular waxes of Eceriferum mutants of Arabidopsis thaliana. Phytochemzstry 33:851–855
Herr JMJ (1982) An analysis of methods for permanently mounting ovules cleared in four-and a-half type clearing fluids. Stain Technol 57:161–169
Heslop-Harrison J (1979) An interpretation of the hydrodynamics of pollen. Am J Bot 66:737–743
Heslop-Harrison J (1987) Pollen germination and pollen tube growth. Int Rev Cytol 107:1–78
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technol 45:115–120
Heslop-Harrison J, Heslop-Harrison Y (1992a) Germination of monocolpate Angiosperm Pollen: Effects of inhibitory factors and the Ca2+-channel blocker, Nifedipine. Ann Bot 69:395–403. http://aob.oxfordjournals.org/content/69/5/395
Heslop-Harrison Y, Heslop-Harrison J (1992b) Germination of monocolpate Angiosperm Pollen: Evolution of the actin cytoskeleton and wall during hydration, activation and tube emergence. Ann Bot 69:385–394. http://aob.oxfordjournals.org/content/69/5/385
Hiroi K, Sone M, Sakazono S, Osaka M, Masuko-Suzuki H, Matsuda T, Suzuki G, Suwabe K, Watanabe M (2013) Time-lapse imaging of self- and cross-pollinations in Brassica rapa. Ann Bot 112:115–122
Hobo T, Suwabe K, Aya K, Suzuki G, Yano K, Ishimizu T, Fujita M, Kikuchi S, Hamada K, Miyano M, Fujioka T, Kaneko F, Kazama T, Mizuta Y, Takahashi H, Shiono K, Nakazono M, Tsutsumi N, Nagamura Y, Kurata N, Watanabe M, Matsuoka M (2008) Various spatiotemporal expression profiles of anther-expressed genes in rice. Plant Cell Physiol 49:1417–1428
Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK (1997) Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9:1999–2010
Iwano M, Shiba H, Miwa T, Che FS, Takayama S, Nagai T, Miyawaki A, Isogai A (2004) Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis. Plant Physiol 136:3562–3571
Iwano M, Shiba H, Matoba K, Miwa T, Funato M, Entani T, Nakayama P, Shimosato H, Takaoka A, Isogai A, Takayama S (2007) Actin dynamics in papilla cells of Brassica rapa during self- and cross-pollination. Plant Physiol 144:72–81
Jayaprakash P, Sarla N (2001) Development of an improved medium for germination of Cajanus cajan (L.) Millsp. pollen in vitro. J Exp Bot 52:851–855
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, Lee J, Kim C, Jang S, Yang K, Nam J, An K, Han MJ, Sung RJ, Choi HS, Yu JH, Choi JH, Cho SY, Cha SS, Kim SI, An G (2000) T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22:561–570
Jeong DH, An S, Kang HG, Moon S, Han JJ, Park S, Lee HS, An K, An G (2002) T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol 130:1636–1644
Jorgensen JH (1992) Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 63:141–152
Kariya K (1989) Sterility caused by cooling treatment at the flowering stage in rice plants. Jpn J Crop Sci 58:96–102
Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U (2010) Conserved molecular components for pollen tube reception and fungal invasion. Science 330:968–971
Kim DH, Eu YJ, Yoo CM, Kim YW, Pih KT, Jin JB, Kim SJ, Stenmark H, Hwang I (2001) Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13:287–301
Kim MC, Lee SH, Kim JK, Chun HJ, Choi MS, Chung WS, Moon BC, Kang CH, Park CY, Yoo JH, Kang YH, Koo SC, Koo YD, Jung JC, Kim ST, Schulze-Lefert P, Lee SY, Cho MJ (2002a) Mlo, a modulator of plant defense and cell death, is a novel calmodulin-binding protein. Isolation and characterization of a rice Mlo homologue. J Biol Chem 277:19304–19314
Kim MC, Panstruga R, Elliott C, Muller J, Devoto A, Yoon HW, Park HC, Cho MJ, Schulze-Lefert P (2002b) Calmodulin interacts with MLO protein to regulate defence against mildew in barley. Nature 416:447–451
Kim SR, Lee DY, Yang JI, Moon S, An G (2009) Cloning vectors for rice. J Plant Biol 52:73–78
Koch KE, Wu Y, Xu J (1996) Sugar and metabolic regulation of genes for sucrose metabolism: potential influence of maize sucrose synthase and soluble invertase responses on carbon partitioning and sugar sensing. J Exp Bot 47:1179–1185
Liu Q, Zhu H (2008) Molecular evolution of the MLO gene family in Oryza sativa and their functional divergence. Gene 409:1–10
Ma JF, Liu ZH, Chu CP, Hu ZY, Wang XL, Zhang XS (2012) Different regulatory processes control pollen hydration and germination in Arabidopsis. Sex Plant Reprod 25:77–82
Matoh T, Ken-ichi I, Ohno K, Azuma J-i (1993) Isolation and characterization of a boron-polysaccharide complex from Radish roots. Plant Cell Physiol 34:639–642. http://pcp.oxfordjournals.org/content/34/4/639.full.pdf
Matoh T, Takasaki M, Takabe K, Kobayashi M (1998) Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol 39:483–491 doi:10.1093/oxfordjournals.pcp.a029395
Mayfield JA, Preuss D (2000) Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nat Cell Biol 2:128–130
Pacini E (1990) Harmomegathic characters of Pteridophyta spores and Spermatophyta pollen. Plant Syst Evol Suppl 5:53–69
Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK (1994) Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. Plant Cell 6:1815–1828
Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol 174:160–173
Preuss D, Lemieux B, Yen G, Davis RW (1993) A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev 7:974–985
Sarker RH, Elleman CJ, Dickinson HG (1988) Control of pollen hydration in Brassica requires continued protein synthesis, and glycosylation in necessary for intraspecific incompatibility. Proc Natl Acad Sci USA 85:4340–4344
Schulze-Lefert P, Panstruga R (2003) Establishment of biotrophy by parasitic fungi and reprogramming of host cells for disease resistance. Annu Rev Phytopathol 41:641–667
Simons G et al (1997) AFLP-based fine mapping of the Mlo gene to a 30-kb DNA segment of the barley genome. Genomics 44:61–70
Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51:49–81
Steinhorst L, Kudla J (2013) Calcium—a central regulator of pollen germination and tube growth. Biochim Biophys Acta 1833:1573–1581
Tanaka M, Fujiwara T (2008) Physiological roles and transport mechanisms of boron: perspectives from plants. Pflugers Arch 456:671–677
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J 11:1187–1194
Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392:818–821
Yi J, An G (2013) Utilization of T-DNA Tagging Lines in Rice. J Plant Biol 56:85–90
Zhang DB, Wilson ZA (2009) Stamen specification and anther development in rice. Chin Sci Bull 54:2342–2353
Zheng L, Baumann U, Reymond JL (2004) An efficient one-step site-directed and site-saturation mutagenesis. Protoc Nucleic Acids Res 32:e115
Acknowledgments
We thank Kyungsook An and Sunghae Hong for managing the transgenic lines. This work was supported in part by grants from the Next-Generation BioGreen 21 Program (No. PJ008215), Rural Development Administration, Republic of Korea; the Basic Research Promotion Fund, Republic of Korea (NRF-2007-0093862); Kyung Hee University (20130214) to G.A.; and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2013R1A6A3A01026391) to J. Y.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Venkatesan Sundaresan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, J., An, S. & An, G. OsMLO12, encoding seven transmembrane proteins, is involved with pollen hydration in rice. Plant Reprod 27, 169–180 (2014). https://doi.org/10.1007/s00497-014-0249-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-014-0249-8