Abstract
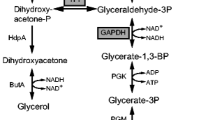
The relationship between the metabolic flux and the activities of the pyruvate branching enzymes of Rhizopus oryzae As 3.2686 during l-lactate fermentation was investigated using the perturbation method of aeration. The control coefficients for five enzymes, pyruvate dehydrogenase (PDH), pyruvate carboxylase (PC), pyruvate decarboxylase (PDC), lactate dehydrogenase (LDH), and alcohol dehydrogenase (ADH), were calculated. Our results indicated significant correlations between PDH and PC, PDC and LDH, PDC and ADH, LDH and ADH, and PDC and PC. It also appeared that PDH, PC, and LDH strongly controlled the l-lactate flux; PDH and ADH strongly controlled the ethanol flux; while PDH and PC strongly controlled the acetyl coenzyme A flux and the oxaloacetate flux. Further, the flux control coefficient curves indicated that the control of the system gradually transferred from PDC to PC during the steady state. Therefore, PC was the key rate-limiting enzyme that controls the flux distribution.


Similar content being viewed by others
References
Huang LP, Jin B, Lant P (2005) Direct fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. Bioprocess Biosyst Eng 27:229–238
Thongchul N, Navankasattusas S, Yang ST (2010) Production of lactic acid and ethanol by Rhizopus oryzae integrated with cassava pulp hydrolysis. Bioprocess Biosyst Eng 33:407–416
Chotisubha-anandha N, Thitiprasert S, Tolieng V, Thongchul N (2011) Improved oxygen transfer and increased L-lactic acid production by morphology control of Rhizopus oryzae in a static bed bioreactor. Bioprocess Biosyst Eng 34:163–172
Phrueksawan P, Kulpreecha S, Sooksai S, Thongchul N (2012) Direct fermentation of L (+)-lactic acid from cassava pulp by solid state culture of Rhizopus oryzae. Bioprocess Biosyst Eng 35:1429–1436
Stephanopoulos GN, Aristos AA, Nielsen J (1998) Metabolic engineering: principles and methodologies. Academic Press, San Diego
Nagamori E, Shimizu K, Fujita H, Tokuhiro K, Ishida N, Takahashi H (2013) Metabolic flux analysis of genetically engineered Saccharomyces cerevisiae that produces lactate under micro-aerobic conditions. Bioprocess Biosyst Eng 36:1261–1265
Pitkänen JP, Aristidou A, Salusjärvi L, Ruohonen L, Penttilä M (2003) Metabolic flux analysis of xylose metabolism in recombinant Saccharomyces cerevisiae using continuous culture. Metab Eng 5:16–31
Bai DM, Zhao XM, Li XG, Xu SM (2004) Strain improvement of Rhizopus oryzae for over-production of L(+)-lactic acid and metabolic flux analysis of mutants. Biochem Eng J 18:41–48
Heijnen JJ, van Gulik WM, Shimizu H, Stephanopoulos G (2004) Metabolic flux control analysis of branch points: an improved approach to obtain flux control coefficients from large perturbation data. Metab Eng 6:391–400
Conradie R, Westerhoff HV, Rohwer JM, Hofmeyr J-HS, Snoep JL (2006) Summation theorems for flux and concentration control coefficients of dynamic systems. IEE Proc Syst Biol 153:314–317
Kurata H, Zhao QY, Okuda R, Shimizu K (2007) Integration of enzyme activities into metabolic flux distributions by elementary mode analysis. BMC Syst Biol 1:31
Ouattara DA, Prot JM, Bunescu A, Dumas ME, Elena-Herrmann B, Leclerc E, Brochot C (2012) Metabolomics-on-a-chip and metabolic flux analysis for label-free modeling of the internal metabolism of HepG2/C3A cells. Mol BioSyst 8:1908–1920
Nielsen J, Jørgensen HS (1995) Metabolic control analysis of the penicillin biosynthetic pathway in a high-yielding strain of Penicillium chrysogenum. Biotechnol Prog 11:299–305
Shiraishi F, Sriyudthsak K, Suzuki Y (2010) Calculation errors of time-varying flux control coefficients obtained from elasticity coefficients by means of summation and connectivity theorems in metabolic control analysis. Math Biosci 223:105–114
Preller A, Quiroga D, Wilson CAM, Ureta T (2007) In vivo determination of the flux control coefficients of the enzymes involved in glycogen synthesis. FEBS J 274:243
Ureta T, Preller A, Wilson C (2008) In vivo flux control coefficient of glycogen synthase in frog oocytes. FEBS J 275:435
Sarıyar B, Perk S, Akman U, Hortaçsu A (2006) Monte Carlo sampling and principal component analysis of flux distributions yield topological and modular information on metabolic networks. J Theoret Biol 242:389–400
Quarato G, Piccoli C, Scrima R, Capitanio N (2011) Variation of flux control coefficient of cytochrome c oxidase and of the other respiratory chain complexes at different values of protonmotive force occurs by a threshold mechanism. BBA Bioenerg 1807:1114–1124
Salusjärvi L, Poutanen M, Pitkänen JP, Koivistoinen H, Aristidou A, Kalkkinen N, Ruohonen L, Penttilä M (2003) Proteome analysis of recombinant xylose-fermenting Saccharomyces cerevisiae. Yeast 20:295–314
Li XF, Qu RN (2012) Parameter identification and terminal steady-state optimization for S system in microbial continuous fermentation. Int J Biomath 5:73–83
Hashemi M, Mousavi SM, Razavi SH, Shojaosadati SA (2011) Mathematical modeling of biomass and α-amylase production kinetics by Bacillus sp. in solid-state fermentation based on solid dry weight variation. Biochem Eng J 53:159–164
Zhong S, Qiu YF, Han BB, Zhao JY, Zhu XJ, Chen XX (2011) Detection of serum desmoglein antibody level using enzyme-linked immunosorbent assay (ELISA) for monitoring disease activity in patients with pemphigus vulgaris. Beijing Da Xue Xue Bao 43:14–415
Duan XZ, Wang M, Li HW, Zhuang H, Xu DP, Wang FS (2004) Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol 24:637–646
Harb J, Lara I, Saleh O, Streif J, Khraiwesh B (2011) Treatments that suppress ethylene production or ethylene action modify ADH and AAT gene expression and aroma-related enzyme activities in ‘Delbarde Estivale’ apple: consequences for the aroma profiles of fruit. J Hortic Sci Biotech 86:182–188
Hu RF, Lin L, Liu TJ, Ouyang PK, He BH, Liu SJ (2008) Reducing sugar content in hemicellulose hydrolysate by DNS method: a revisit. J Biobased Mater Bioenerg 2:156–161
Ke W, Zheng Z, Jiang ST, Luo SZ, Wu XF, Yang PZ (2014) The analysis of metabolic flux and enzyme activities of pyruvate branch point of Rhizopus oryzae As 3.2686. J Chem Eng Chin Univ 28:1071–1079 (in Chinese)
Liu RX, Kuang J, Gong Q, Hou XL (2003) Principal component regression analysis with SPSS. Comput Methods Programs Biomed 71:141–147
Bai DM, Fu WM, Zhao XM, Dai HX, Li XG, Xu SM (2002) Optimization of L(+)-Lactic acid fermentation by Rhizopus oryzae R1021 by metabolic flux analysis. J Wuxi Univ Light Ind 21:554–558 (in Chinese)
Skory CD (2004) Lactic acid production by Rhizopus oryzae transformants with modified lactate dehydrogenase activity. Appl Microbiol Biot 64:237–242
Lin H, Vadali RV, Bennett GN, San KY (2004) Increasing the acetyl-CoA pool in the presence of overexpressed phosphoenolpyruvate carboxylase or pyruvate carboxylase enhances succinate production in Escherichia coli. Biotechnol Prog 20:1599–1604
Wu SG, Zhang T, Deng L (2011) Effect of over-expressing of pyruvate carboxylase gene on production of fumarate. Biotechnol Bull 12:175–180 (in Chinese)
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 31071636/31171741) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 2010JYLH0837).
Author information
Authors and Affiliations
Corresponding author
Additional information
W. Ke and S. Chang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ke, W., Chang, S., Chen, X. et al. Metabolic control analysis of l-lactate synthesis pathway in Rhizopus oryzae As 3.2686. Bioprocess Biosyst Eng 38, 2189–2199 (2015). https://doi.org/10.1007/s00449-015-1458-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1458-8