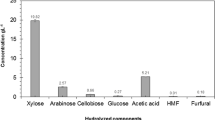
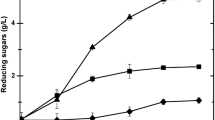
Abstract
Cassava pulp was hydrolyzed with acids or enzymes. A high glucose concentration (>100 g/L) was obtained from the hydrolysis with 1 N HCl at 121 °C, 15 min or with cellulase and amylases. While a high glucose yield (>0.85 g/g dry pulp) was obtained from the hydrolysis with HCl, enzymatic hydrolysis yielded only 0.4 g glucose/g dry pulp. These hydrolysates were used as the carbon source in fermentation by Rhizopus oryzae NRRL395. R. oryzae could not grow in media containing the hydrolysates treated with 1.5 N H2SO4 or 2 N H3PO4, but no significant growth inhibition was found with the hydrolysates from HCl (1 N) and enzyme treatments. Higher ethanol yield and productivity were observed from fermentation with the hydrolysates when compared with those from fermentation with glucose in which lactic acid was the main product. This was because the extra organic nitrogen in the hydrolysates promoted cell growth and ethanol production.







Similar content being viewed by others
References
Nishise H, Fuji A, Ueno M, Vongsuvanlert V, Tani Y (1988) Production of raw cassava starch-digestive glucoamylase by Rhizopus sp. in liquid culture. J Ferment Technol 66:397–402
Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, Mohan R (2000) Biotechnological potential of agro-industrial residues. II: cassava bagasse. Bioresource Technol 74:81–87
Soccol CR, Vandenberghe LPS (2003) Overview of applied solid-state fermentation in Brazil. Biochem Eng J 13:205–218
Sriroth K, Chollakup R, Chotineeranat S, Piyachomkwan K, Oates CG (2000) Processing of cassava waste for improved biomass utilization. Bioresour Technol 71:63–69
Carta FS, Soccol CR, Ramos LP, Fontana JD (1999) Production of fumaric acid by fermentation of enzymatic hydrolysates derived from cassava bagasse. Bioresour Technol 68:23–28
Christen P, Bramorski A, Revah S, Soccol CR (2000) Characterization of volatile compounds produced by Rhizopus strains grown on agro-industrial solid wastes. Bioresour Technol 71:211–215
Kitpreechavanich V, Maneeboon T, Kayano Y, Sakai K (2008) Comparative characterization of l-lactic acid-producing thermotolerant Rhizopus fungi. J Biosci Bioeng 106:541–548
Vink ETH, Rabago KR, Glassner DA, Springs R, O’Corner RP, Gruber PR (2004) The sustainability of NatureWorksTM polylactide polymers and IngeoTM polylactide fibersa: an update of the future. Macromol Biosci 4:551–564
Yu R-C, Hang YD (1989) Kinetics of direct fermentation of agricultural commodities to l(+)-lactic acid by Rhizopus oryzae. Biotechnol Lett 11:597–600
Zhan X, Wang D, Tuinstra MR, Bean S, Seib PA, Sun XS (2003) Ethanol and lactic acid production as affected by sorghum genotype and location. Ind Crop Prod 18:245–255
Aikat K, Bhattacharyya BC (2001) Protease production in solid state fermentation with liquid medium recycling in a stacked plate reactor and in a packed bed reactor by a local strain of Rhizopus oryzae. Process Biochem 36:1059–1068
Huang LP, Jin B, Lant P (2005) Direct fermentation of potato starch wastewater to lactic acid by Rhizopus arrhizus. Bioprocess Biosyst Eng 27:229–238
Ruengruglikit C, Hang YD (2003) l(+)-lactic acid production from corncobs by Rhizopus oryzae NRRL-395. LWT Food Sci Technol 36:573–575
Sun SY, Xu Y (2008) Solid-state fermentation for ‘whole-cell synthetic lipase’ production from Rhizopus chinensis and identification of the functional enzyme. Process Biochem 43:219–224
Taherzadeh MJ, Fox M, Hjorth H, Edebo L (2003) Production of mycelium biomass and ethanol from paper pulp sulfite liquor by Rhizopus oryzae. Bioresour Technol 88:167–177
Tani Y, Fuji A, Nishise H (1988) Production of raw cassava starch-digestive glucoamylase by a 2-deoxyglucose-resistant mutant of Rhizopus sp. J Ferment Technol 66:545–551
Cantarella M, Cantarella L, Gallifuoco A, Spera A, Alfani F (2004) Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol Prog 20:200–206
Zhang ZY, Jin B, Kelly JM (2007) Production of lactic acid from renewable materials by Rhizopus fungi. Biochem Eng J 35:251–263
Thongchul N, Yang S-T (2003) Controlling filamentous fungal morphology by immobilization on a rotating fibrous matrix to enhance oxygen transfer and l(+)-lactic acid production by Rhizopus oryzae. In: Saha BC (ed) Fermentation biotechnology. Oxford University Press, MA, pp 36–51
Lowry OH, Rosenbrough NJ, Farr AL, Randell RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Chamy R, Illanes A, Aroca G, Nunez L (1994) Acid hydrolysis of sugar beet pulp as pretreatment for fermentation. Bioresour Technol 50:149–152
Stryer B (1995) Biochemistry, 4th edn. Freeman and Company, New York
Urbaneja G, Ferrer J, Paez G, Arenas L, Colina G (1996) Acid hydrolysis and carbohydrates characterization of coffee pulp. Renew Energy 9:1041–1044
Malester AI, Green M, Kimchie S, Shelef G (1988) The effect of the neutralizing capacity of cellulosic materials on the kinetics of cellulose dilute acid hydrolysis. Biol Waste 26:115–124
Nichols NN, Sharma LN, Mowery RA, Chambliss CK (2008) Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzym Microb Tech 42:624–630
Agu RC, Amadife AE, Ude CM, Onyia A, Ogu EO, Okafor M (1997) Combined heat treatment and acid hydrolysis of cassava grate waste (CGW) biomass for ethanol production. Waste Manag 17:91–96
Zhu Y, Wu Z, Yang S-T (2002) Butyric acid production from acid hydrolysate of corn fiber by Clostridium tyrobutyricum in a fibrous-bed bioreactor. Process Biochem 38:657–666
Mussatto SI, Marcela F, Milagres AMF, Roberto IC (2008) Effect of hemicelluloses and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzym Microb Tech 43:124–129
Absar N, Zaidul ISM, Takigawa S, Hashimoto N, Matsuura-Endo C, Yamauchi H, Noda T (2009) Enzymatic hydrolysis of potato starches containing different amounts of phosphorus. Food Chem 112:57–62
Kassim EA, El-Shahed AS (1986) Enzymatic and chemical hydrolysis of certain cellulosic materials. Agric Wastes 17:229–233
Chandra M, Kalra A, Sangwan NS, Gaurav SS, Darokar MP, Sangwan RS (2009) Development of a mutant of Trichoderma citrinoviride for enhanced production of cellulases. Bioresour Technol 100:1659–1662
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74:524–534
Woiciechowski AL, Nitsche S, Pandey A, Soccol CR (2002) Acid and enzymatic hydrolysis to recover reducing sugars from cassava bagasse: an economic study. Braz Arch Biol Technol 45:393–400
Buchholz K, Seibel J (2008) Industrial carbohydrate biotransformations. Carbohydr Res 343:1966–1979
Gamez S, Gonzalez-Cabriales JJ, Ramirez JA, Garrote G, Vazquez M (2006) Study of the hydrolysis of sugar cane bagasse using phosphoric acid. J Food Eng 74:78–88
Grzenia DL, Schell DJ, Wickramasinghe SR (2008) Membrane extraction for removal of acetic acid from biomass hydrolysates. J Membr Sci 322:189–195
Qureshi N, Ezeji TC, Ebener J, Dien BS, Cotta MA, Blaschek HP (2008) Butanol production by Clostridium beijerinckii. Part I: use of acid and enzyme hydrolyzed corn fiber. Bioresour Technol 99:5915–5922
Skory CD, Freer SN, Bothast RJ (1998) Production of l-lactic acid by Rhizopus oryzae under oxygen limiting conditions. Biotechnol Lett 20:191–194
Tay A, Yang S-T (2002) Production of l(+)-lactic acid from glucose and starch by immobilized cells of Rhizopus oryzae in a rotating fibrous bed bioreactor. Biotechnol Bioeng 80:1–12
Liu Y, Liao W, Liu C, Chen S (2006) Optimization of l-(+)-lactic acid production using pelletized filamentous Rhizopus oryzae NRRL395. Appl Biochem Biotech 129–132:844–853
Roble ND, Ogbonna JC, Tanaka H (2003) l-lactic acid production from raw cassava starch in a circulating loop bioreactor with cells immobilized in loofa (Luffa cylindrical). Biotechnol Lett 25:1093–1098
John RP, Sukumaran RK, Nampoothiri KM, Pandey A (2007) Statistical optimization of simultaneous saccharification and l(+)-lactic acid fermentation from cassava bagasse using mixed culture of lactobacilli by response surface methodology. Biochem Eng J 36:262–267
Ganguly R, Dwivedi P, Singh RP (2007) Production of lactic acid with loofa sponge immobilized Rhizopus oryzae RBU2-10. Bioresour Technol 98:1246–1251
Acknowledgments
This work has been financially supported by Commission on Higher Education and Thailand Research Fund (Grant number: MRG5080097). Raw cassava pulp was kindly provided by Chonjareon Cassava Mill, Thailand. We thank Sitanan Thitiprasert for assistance in analyzing fermentation samples and Siam Victory Chemicals, Thailand, for technical supports and providing enzyme samples (Genencor products).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thongchul, N., Navankasattusas, S. & Yang, ST. Production of lactic acid and ethanol by Rhizopus oryzae integrated with cassava pulp hydrolysis. Bioprocess Biosyst Eng 33, 407–416 (2010). https://doi.org/10.1007/s00449-009-0341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-009-0341-x