Abstract
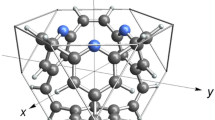
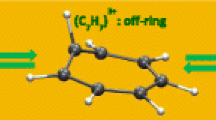
A hydrocarbon molecule, having a truncated tetrahedron shape with a suitable size for the storage of a hydrogen molecule, is designed using quantum chemical methods. The molecule consists of four benzene rings bridged by six vinylene groups at the 1, 3, and 5 carbon positions of each ring, and has a stoichiometry of C36H24. The molecular geometry optimized under T d symmetry by the B3LYP/cc-pVTZ method shows no imaginary frequencies. The size of the molecular cavity, measured by the distance between opposite vinylene groups, is 8.0 Å. The cavity has four openings along each tetrahedron face. The radius of the opening is approximately 2 Å. The system interacting with a hydrogen molecule is optimized by the MP2/cc-pVTZ method. The interaction energy is evaluated by an extrapolation method through increasing the basis set size of the hydrogen molecule from the cc-pVTZ to the cc-pV6Z with counterpoise corrections. The hydrogen molecule enters the opening by overcoming an energy barrier of +730 meV and locates at the center of the cavity with a binding energy of −140 meV. The high barrier arises from the small size of the opening. The binding energy is three times larger than that of a graphite surface and may allow hydrogen storage at milder temperatures and pressures than those required with graphite.






Similar content being viewed by others
References
Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan, Sect. 3.3 Hydrogen Storage, 2012. http://energy.gov/sites/prod/files/2014/03/f12/storage.pdf, Accessed 12 Sep 2016
K.M. Thomas, Catal. Today 120, 389 (2007)
B. Bogdanović, M. Schwickardi, J. Alloys. Compd. 253–254, 1(1997)
L. Mattera, F. Rosatelli, C. Salvo, F. Tommasini, U. Valbusa, G. Vidali, Surf. Sci 93, 515 (1980)
P. Bénard, R. Chahine, Langmuir 17, 1950 (2001)
S. Patchkovskii, J.S. Tse, S.N. Yurchenko, L. Zhchkov, T. Heine, G. Seifert, Proc. Nat. Acad. Sci. USA 102, 10439 (2005)
H. Cheng, A.C. Cooper, G.P. Pez, M.K. Kostov, P. Piotrowski, S.J. Stuart, J. Phys. Chem. B 109, 3780 (2005)
B. Kuchta, L. Firlej, P. Pfeifer, C. Wexler, Carbon 48, 223 (2010)
S. Ishikawa, T. Yamabe, Appl. Phys. A 114, 1339 (2014)
S. Ishikawa, T. Yamabe, Appl. Phys. A 119, 1365 (2015)
J.H. Conway, H. Burgiel, C. Goodman-Strass, The Symmetries of Things, 1st edn. (CRC Press, 2008), pp. 283–353
Gaussian 09, Revision E.01, M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. J.B. Farkas, Foresman, J.V. Ortiz, D.J. J. Cioslowski, Fox. (Gaussian, Inc., Wallingford CT, 2009)
C. Møller, M.S. Plesset, Phys. Rev 46, 618 (1934)
J.A. Pople, K. Raghavachari, H.B. Schlegel, J.S. Binkley. Int. J. Quantum Chem., Quant. Chem. Symp S13, 225 (1979)
T.H. Dunning Jr, J. Chem. Phys. 90, 1007 (1989)
A.K. Wilson, T. van Mourik, T.H. Dunning Jr., J. Mol, Struct. Theochem 388, 339 (1996)
T.M. Klapötke, A. Schulz, in Quantum Chemical Methods in Main-Group Chemistry (Wiley, Chichester, 1998), pp. 115–118
S.F. Boys, F. Bernardi, Mol. Phys 19, 553 (1970)
A.D. Becke, J. Chem. Phys. 98, 5648 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
A. Bondi, J. Phys. Chem 68, 441 (1964)
O. Hübner, A. Glöss, M. Fichtner, W. Klopper, J. Phys. Chem. A 108, 3019 (2004)
S. Ishikawa, T. Yamabe, Appl. Phys. A 99, 29 (2010)
I.N. Levine, Quantum Chemistry, 4th edn, (Prentice Hall, Englewood Cliffs, 1991), pp. 603
M.W. Feyereisen, D. Feller, A.A. Dixon, J. Phys. Chem 100, 2993 (1996)
S.K. Bhatia, A.L. Myers, Langmuir 22, 1688 (2006)
A.R. Leach, Molecular Modelling: Principles and Applications, 2nd edn, (Pearson Education Ltd., Harlow 2001), pp. 221
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix
Appendix
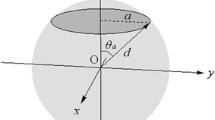
Here, we show the detailed form of the potential function of a hydrogen molecule bound in a carbon sphere of radius d with a circular opening of radius a [9, 10]. The origin of the system is set at the sphere center and the z axis is drawn running through the centers of the sphere and the opening. The potential energy curve of a hydrogen molecule moving along the z axis is given by:
where θ a , D, and d e denote the polar angle of the opening edge (\({{\theta }_{a}}={{\sin }^{-1}}a/d\)), the potential depth, and the optimum sphere radius, respectively. The lowest energy (−D) is obtained when the hydrogen molecule is located at the center of the sphere (z = 0) if d = d e and a = 0. The D and d e are given by:
where ρ s denotes the density of the carbon surface. The \({{\epsilon }_{\text{C}-{{\text{H}}_{2}}}}\)and \({{\sigma }_{\text{C}-{{\text{H}}_{2}}}}\) are, respectively, the energy and distance parameters of the Lennard–Jones potential between a carbon atom and a hydrogen molecule. The hydrogen scattering experiment from the graphite surface determined their values: \({{\epsilon }_{\text{C}-{{\text{H}}_{2}}}}\)= 3.89 meV and \({{\sigma }_{\text{C}-{{\text{H}}_{2}}}}\)= 2.89 Å [4]. Using the graphite surface density (0.382 Å−2) for ρ s , we obtain D = 203 meV and d e = 3.37 Å.
Rights and permissions
About this article
Cite this article
Ishikawa, S., Yamabe, T. Theoretical study of hydrogen storage in a truncated tetrahedron hydrocarbon. Appl. Phys. A 123, 119 (2017). https://doi.org/10.1007/s00339-016-0726-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-016-0726-z