Abstract
Recent reports have revealed that cynomolgus macaques obtained from different geographic origins may be more or less suitable for particular studies depending on the specific question(s) being addressed, e.g. Mauritian cynomolgus macaques are particularly suitable for detailed immunological studies against a limited genetic background while less conserved populations may be more appropriate to predict breadth of vaccine coverage in the genetically diverse human population. We have characterised MHC haplotypes in 90 Indonesian cynomolgus macaques using microsatellite and reference strand conformational analysis. Thirty unique haplotypes were defined in the cohort, emphasising the high degree of diversity in this population of cynomolgus macaques. The majority of haplotypes were present at a frequency of ≤6%. Transcription profiles indicated that each haplotype was associated with two to eight transcribed class I alleles. The results corroborate previous reports of the extensive MHC diversity of Indonesian cynomolgus macaques and provide additional data to inform colony management decisions. Further, definition of the MHC diversity of the population satisfies one of the prerequisites to MHC association studies and detailed immunological investigations in this outbred non-human primate species.
Similar content being viewed by others
Introduction
Macaque monkeys (Macaca spp.), particularly cynomolgus, rhesus and pigtail macaques, are widely used in infectious disease pathogenesis models (Feichtinger et al. 1990; Dittmer et al. 1996; Maggiorella et al. 1998; Montgomery et al. 1999; Nalca et al. 2010; Sharpe et al. 2010), vaccine development (Gotch et al. 1991; Negri et al. 2004; Berry et al. 2007; Kawada et al. 2007; Maggiorella et al. 2007; Weiss et al. 2007; Jiang et al. 2009; Mudd et al. 2010) and organ transplant research (reviewed in Hale et al. 2005). Recently, the demand for macaques of defined MHC genotype has increased in light of numerous reports demonstrating differential control of pathogens, most notably SIV, in animals expressing particular MHC class I alleles and/or haplotypes (Muhl et al. 2002; Mothe et al. 2003; Loffredo et al. 2007; Florese et al. 2008; Sauermann et al. 2008; Mee et al. 2009b, 2010; Aarnink et al. 2011).
Cynomolgus macaque breeding facilities have typically been established using animals from a number of locations including Indonesia, Malaysia, China, the Philippines and Mauritius. Mauritian cynomolgus macaques (MCM) have been shown to be particularly valuable for detailed immunological studies due to the remarkably restricted MHC diversity of feral and captive-bred populations (Wiseman et al. 2007; Mee et al. 2009a). While MCM are clearly valuable as a homogenous population for biomedical studies, many primate facilities also maintain non-Mauritian macaque colonies and in certain cases, it may be more appropriate to perform pathogenesis or vaccine studies in a non-human primate species more representative of the outbred human population. We therefore investigated the degree of MHC diversity in a population of cynomolgus macaques of predominantly Indonesian origin in a UK breeding colony to determine whether the high degree of MHC diversity reported in other colonies of Indonesian macaques (Pendley et al. 2008; Kita et al. 2009; Otting et al. 2009) was reproduced and to provide additional genetic data to inform colony management decisions. Using microsatellite and reference strand conformational analysis (RSCA), we identified 30 distinct haplotype configurations among 90 macaques. The data confirm and extend recent reports of the extensive MHC diversity in this population, facilitate genetics-led colony management decisions and provide a foundation for detailed immunological analyses using Indonesian cynomolgus macaques.
Methods
Animals
Ninety cynomolgus macaques of primarily Indonesian origin housed at a UK breeding facility were sampled. The colony was established from approximately 100 founder animals, and animals in the current study represent the third to fifth generation descendants of the founders. Records indicate that limited mixing of animals of two different geographic origins occurred prior to the establishment of the extant ‘Indonesian’ breeding colonies. These colonies have subsequently been housed separately from Mauritian macaque colonies. The study cohort included 73 cynomolgus macaques from four newly established, specific pathogen-free colonies (Mee et al. 2009c) each comprising 14–22 macaques. Where available, parental samples were analysed to infer or confirm haplotypes. Animals were housed and maintained in accordance with UK Home Office guidelines for care and maintenance of non-human primates.
DNA extraction
Blood samples from all animals were taken into EDTA. DNA was extracted by phenol–chloroform extraction and isopropanol precipitation, resuspended in molecular-grade water and diluted to 10 ng/μl.
RNA extraction and cDNA synthesis
RNA was extracted from 1.5 ml fresh EDTA-treated blood using the QIAamp RNA blood mini kit (Qiagen, West Sussex, UK) according to the method recommended by the manufacturer. Approximately 1 μg of RNA was converted to cDNA using the Accuscript High Fidelity 1st Strand cDNA Synthesis Kit (Agilent Technologies, Stockport, UK) and the provided oligo(dT) primer.
Microsatellite analysis
Microsatellite analysis was performed using a previously described method (Wiseman et al. 2007).
Reference strand conformational analysis
Amplicons spanning 305 bp of exons 2–3 of the class I heavy chain gene were prepared from cDNA as described previously (Mee et al. 2009a). A 1.5 μl volume of standard or sample was mixed with 3 μl diluted fluorescent-labelled referenced strand and denatured at 95°C for 5 min. The reaction was then cooled at −1°C/s to 55°C, held for 5 min and cooled at −1°C/s to 15°C. Samples were refrigerated until use. Immediately prior to use, the sample was diluted 1:5 in ultrapure water and 2 μl was added to 8 μl water containing 0.08 μl ROX-ET900 size ladder (GE Healthcare, Buckinghamshire, UK). Samples were separated on an ABI 3130 genetic analyser, using a 36-cm capillary filled with 4% (w/w) non-denaturing conformational analysis polymer buffered with 1× running buffer containing EDTA (Applied Biosystems). Samples were electrokinetically injected at 15 kV for 15 s and separated at 4 kV for 60 min at a constant temperature of 30°C. Apparent mobility was defined in arbitrary units corresponding to the scan number at which the peaks of the ROX-ET900 ladder were detected. Mobility bins for each FLR/allele combination were defined using Genemapper v4.0 (Applied Biosystems). All samples were re-examined manually to ensure correct calling of peaks.
Data analysis
Haplotypes were initially characterised by identifying microsatellite profiles that extended across all 18 markers and were present in three or more animals. The presence of a number of heterozygote animals carrying Mauritian haplotypes facilitated the definition of the correct phase of a number of haplotypes. For haplotypes where only two macaques displayed identical microsatellite patterns, parental DNA samples were analysed to confirm inheritance and validity of data. Unassigned haplotypes were then re-examined to identify those sharing at least 14 of 18 markers with an established haplotype. RSCA was performed on RNA samples where available (88% of macaques); if identical RSCA profiles were observed, the haplotypes were considered to be identical at the class I allele transcript level. Finally, recombinant haplotypes were defined where three or more consecutive markers corresponding to distinct haplotypes were present on the same chromosome.
Results
Microsatellite analysis defines at least 30 haplotypes in Indonesian cynomolgus macaques
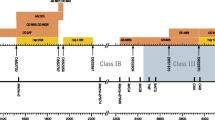
We determined MHC haplotypes in 90 Indonesian cynomolgus macaques using a panel of microsatellite markers previously reported to be highly informative for the genotyping of Mauritian cynomolgus macaque populations. Thirty-five unique microsatellite profiles were identified with each profile present in at least two macaques (Supplemental Fig. 1). Due to the mixing which occurred prior to the establishment of the Indonesian colonies, five of these profiles which accounted for 12% of all chromosomes studied corresponded to MHC haplotypes previously described in Mauritian cynomolgus macaque populations (Wiseman et al. 2007; Mee et al. 2009a). Analysis of heterozygote animals carrying Mauritian haplotypes allowed for the initial determination of the phase of a number of Indonesian haplotypes. Of the 30 Indonesian haplotypes identified (Table 1), most were found at low frequency (≤6% of chromosomes), emphasising the high level of diversity among this population of macaques (Fig. 1). Twenty animals possessed one haplotype to which a unique microsatellite profile could not be assigned due to the lack of additional animals with a matching profile. Thirty-four chromosomes (19% of all chromosomes) appeared to carry recombinant haplotypes as evidenced by the combination of microsatellite profiles corresponding to two or more of the major haplotypes and/or unassigned microsatellite patterns. Only four of these recombinants appeared to be derived from recombination of Mauritian and Indonesian haplotypes, the remainder were Mauritian/Mauritian, Indonesian/Indonesian or Indonesian/unassigned (Supplemental Fig. 1). In two cases, inheritance of recombinant haplotypes was confirmed by pedigree analysis [animals 548FBG (dam), 548FBGA (offspring) and 980ABAE (sire), Supplemental Fig. 1].
Frequency of novel MHC haplotypes among a cohort of 90 Indonesian cynomolgus macaques. Bars indicate the number of animals carrying each haplotype. White fill indicates animals where the haplotype was identified by microsatellite analysis alone. Black fill indicates animals where haplotype was identified by microsatellite analysis and confirmed by RSCA. ND not determined, haplotype could not be confirmed in two or more animals; RC recombinant haplotype; MCM previously described Mauritian cynomolgus macaque haplotypes; IND novel Indonesian cynomolgus macaque haplotypes
Analysis of RSCA profiles confirms distinct repertoires of class IA and B alleles
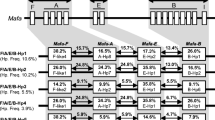
In several cases different microsatellite allele sizes were associated with the same haplotype, e.g. two IND 3 profiles were identified (Table 1), each of which shared 15 marker sizes but differed at three. We confirmed that such haplotypes carried common MHC class I genes by analysing the class I transcript profile using RSCA (representative RSCA profiles are shown in Fig. 2). Haplotypes with divergent microsatellite profiles were classed as identical if no more than four microsatellites exhibited different allele sizes and similar RSCA profiles could be identified in all animals carrying that haplotype. Where only two macaques were shown to display the same microsatellite pattern and RSCA profile, microsatellite analysis was performed on parental animals to confirm inheritance. Inheritance was confirmed for 17 haplotypes in this way; the remaining 13 haplotypes were all detected in at least three unrelated animals. RSCA identified two to eight distinct peaks per haplotype, consistent with the numbers of class I alleles previously reported in Indonesian populations of cynomolgus macaques (two to eight transcribed alleles per animal, Pendley et al. 2008; Otting et al. 2009). Within the 30 novel haplotypes, none shared more than two class I alleles as determined by RSCA. Thus, despite a small number of shared alleles, each haplotype appears to carry a largely distinct repertoire of class I alleles.
Comparison of RSCA profiles in selected animals 019GG (top) and 019GGB (bottom). Arrows denote alleles shared between both animals, which also share one MHC haplotype as defined by microsatellite analysis. Residual FLR homoduplex is present in both samples at an apparent mobility of approximately 1,110
Discussion
We have investigated MHC diversity in a large cohort of Indonesian macaques housed in a UK breeding facility and identified at least 30 distinct ‘Indonesian’ MHC haplotype configurations among 90 macaques. The actual number of haplotypes likely exceeds this number since a further 20 microsatellite patterns could not be confirmed due to the absence of samples from related animals. Several putative additional haplotypes could be identified in animals carrying one assigned haplotype (Supplemental Fig. 1), however for consistency we did not consider these to be authentic haplotypes unless they met the criteria described in the “Methods” section, i.e. present in at least three animals or confirmed by inheritance. Since the animals in this study were selected from active breeding colonies, it will be possible to confirm these patterns in the future using samples from offspring. As the colony is self-sustaining, the entire genetic diversity of the breeding population can be described and subsequently manipulated by selective breeding of animals with the most appropriate genotypes. The results confirm previous reports (Pendley et al. 2008; Kita et al. 2009; Otting et al. 2009) that Indonesian macaques exhibit a high degree of MHC diversity and are more outbred than the previously characterised MCM. Notably, the data also indicate that a high degree of diversity is maintained within our colonies despite the recent establishment of SPF colonies by selective regrouping of animals (Mee et al. 2009c). RSCA profiles suggested that between two and eight class I alleles are transcribed from each haplotype, though the actual number may be higher since some alleles are likely transcribed at a level below the detection limit of RSCA and different alleles may co-migrate when hybridised to the fluorescent reference strand. More sensitive technology, such as high-throughput pyrosequencing, would provide a more complete profile of transcribed class I alleles, but such an approach was outside the scope of the present study.
This extensive diversity makes Indonesian cynomolgus macaques valuable for biomedical research where the use of an outbred population is desirable. Pathogen association studies at the haplotype level, similar to those performed in MCM, are unlikely to be feasible in cohorts of Indonesian cynomolgus macaques due to the low frequency of shared haplotypes. It is likely, however, that several of the haplotypes share MHC class I alleles. The use of allele-specific PCR in conjunction with recently developed pyrosequencing methods for detailed identification of transcribed class I alleles (Wiseman et al. 2009; Budde et al. 2010) will permit the assignment of individual alleles to each haplotype and facilitate association studies to identify those alleles relevant to immune responses against pathogens. The utility of microsatellite analysis for rapid genotyping of animals will enable the identification of animals carrying rare haplotypes, within this and other cohorts, that represent useful candidates for the identification of novel MHC alleles.
In addition to the novel Indonesian MHC haplotypes, five haplotypes identical to those previously reported in MCM were identified. Breeding records indicate that animals of unknown geographic origin, but likely MCM, were mixed with our Indonesian breeding colonies in the past, therefore these MCM haplotypes most likely reflect genetic contamination rather than shared ancestral haplotypes. Though the latter is a possibility, previous reports have suggested only distantly related MHC haplotypes between cynomolgus macaques from Mauritius and Indonesia or the Philippines (Campbell et al. 2008; Pendley et al. 2008). Furthermore, analysis of a separate cohort of animals derived from the same breeding colony confirmed the presence of mitochondrial DNA sequences characteristic of MCM in addition to those from Indonesia (Rose et al., unpublished data). The characterisation of the colony MHC diversity presented herein and the relative ease of microsatellite-based genotyping will facilitate the exclusion of these contaminating MCM MHC haplotypes by selective breeding if required.
In summary, we have characterised MHC haplotypes in a cohort of 90 Indonesian cynomolgus macaques. The results are consistent with previous reports of a high degree of genetic diversity in this population of macaques and add to the growing corpus of data on the MHC genetics of this species. Our findings provide a foundation for improved genetic management of macaque breeding colonies and pathogen association studies in an outbred non-human primate species.
References
Aarnink A, Dereuddre-Bosquet N, Vaslin B, Le Grand R, Winterton P, Apoil PA, Blancher A (2011) Influence of the MHC genotype on the progression of experimental SIV infection in the Mauritian cynomolgus macaque. Immunogenetics 63:267–274
Berry N, Stebbings R, Brown S, Christian P, Thorstensson R, Ahmed RK, Davis L, Ferguson D, D'Arcy N, Elsley W, Hull R, Lines J, Wade-Evans A, Stott J, Almond N (2007) Immunological responses and viral modulatory effects of vaccination with recombinant modified vaccinia virus Ankara (rMVA) expressing structural and regulatory transgenes of simian immunodeficiency virus (SIVmac32H/J5M). J Med Primatol 36:80–94
Budde ML, Wiseman RW, Karl JA, Hanczaruk B, Simen BB, O'Connor DH (2010) Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics 62:773–780
Campbell KJ, Detmer AM, Karl JA, Wiseman RW, Blasky AJ, Hughes AL, Bimber BN, O'Connor SL, O'Connor DH (2008) Characterization of 47 MHC class I sequences in Filipino cynomolgus macaques. Immunogenetics 61(3):177–187
Dittmer U, Petry H, Stahl-Hennig C, Nisslein T, Spring M, Luke W, Bodemer W, Kaup FJ, Hunsmann G (1996) T cell apoptosis in human immunodeficiency virus type 2- and simian immunodeficiency virus-infected macaques. J Gen Virol 77:2433–2436
Feichtinger H, Putkonen P, Parravicini C, Li SL, Kaaya EE, Bottiger D, Biberfeld G, Biberfeld P (1990) Malignant lymphomas in cynomolgus monkeys infected with simian immunodeficiency virus. Am J Pathol 137:1311–1315
Florese RH, Wiseman RW, Venzon D, Karl JA, Demberg T, Larsen K, Flanary L, Kalyanaraman VS, Pal R, Titti F, Patterson LJ, Heath MJ, O'Connor DH, Cafaro A, Ensoli B, Robert-Guroff M (2008) Comparative study of Tat vaccine regimens in Mauritian cynomolgus and Indian rhesus macaques: influence of Mauritian MHC haplotypes on susceptibility/resistance to SHIV(89.6P) infection. Vaccine 26:3312–3321
Gotch FM, Hovell R, Delchambre M, Silvera P, McMichael AJ (1991) Cytotoxic T-cell response to simian immunodeficiency virus by cynomolgus macaque monkeys immunized with recombinant vaccinia virus. AIDS 5:317–320
Hale DA, Dhanireddy K, Bruno D, Kirk AD (2005) Induction of transplantation tolerance in non-human primate preclinical models. Philos Trans R Soc Lond B Biol Sci 360:1723–1737
Jiang G, Shi M, Conteh S, Richie N, Banania G, Geneshan H, Valencia A, Singh P, Aguiar J, Limbach K, Kamrud KI, Rayner J, Smith J, Bruder JT, King CR, Tsuboi T, Takeo S, Endo Y, Doolan DL, Richie TL, Weiss WR (2009) Sterile protection against Plasmodium knowlesi in rhesus monkeys from a malaria vaccine: comparison of heterologous prime boost strategies. PLoS One 4:e6559
Kawada M, Tsukamoto T, Yamamoto H, Takeda A, Igarashi H, Watkins DI, Matano T (2007) Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J Virol 81:5202–5211
Kita YF, Hosomichi K, Kohara S, Itoh Y, Ogasawara K, Tsuchiya H, Torii R, Inoko H, Blancher A, Kulski JK, Shiina T (2009) MHC class I A loci polymorphism and diversity in three Southeast Asian populations of cynomolgus macaque. Immunogenetics 61:635–648
Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, Lifson JD, Carrington M, Watkins DI (2007) Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol 81:8827–8832
Maggiorella MT, Monardo F, Koanga-Mogtomo ML, Cioe L, Sernicola L, Corrias F, Baroni CD, Verani P, Titti F (1998) Detection of infectious simian immunodeficiency virus in B- and T-cell lymphomas of experimentally infected macaques. Blood 91:3103–3111
Maggiorella MT, Sernicola L, Crostarosa F, Belli R, Pavone-Cossut MR, Macchia I, Farcomeni S, Tenner-Racz K, Racz P, Ensoli B, Titti F (2007) Multiprotein genetic vaccine in the SIV-Macaca animal model: a promising approach to generate sterilizing immunity to HIV infection. J Med Primatol 36:180–194
Mee ET, Badhan A, Karl JA, Wiseman RW, Cutler K, Knapp LA, Almond N, O'Connor DH, Rose NJ (2009a) MHC haplotype frequencies in a UK breeding colony of Mauritian cynomolgus macaques mirror those found in a distinct population from the same geographic origin. J Med Primatol 38:1–14
Mee ET, Berry N, Ham C, Aubertin A, Lines J, Hall J, Stebbings R, Page M, Almond N, Rose NJ (2010) Mhc haplotype M3 is associated with early control of SHIVsbg infection in Mauritian cynomolgus macaques. Tissue Antigens 76:223–229
Mee ET, Berry N, Ham C, Sauermann U, Maggiorella MT, Martinon F, Verschoor EJ, Heeney JL, Le Grand R, Titti F, Almond N, Rose NJ (2009b) Mhc haplotype H6 is associated with sustained control of SIVmac251 infection in Mauritian cynomolgus macaques. Immunogenetics 61:327–339
Mee ET, Murrell CK, Watkins J, Almond N, Cutler K, Rose NJ (2009c) Low rates of transmission of SRV-2 and STLV-I to juveniles in a population of Macaca fascicularis facilitate establishment of specific retrovirus-free colonies. J Med Primatol 38:160–170
Montgomery MM, Dean AF, Taffs F, Stott EJ, Lantos PL, Luthert PJ (1999) Progressive dendritic pathology in cynomolgus macaques infected with simian immunodeficiency virus. Neuropathol Appl Neurobiol 25:11–19
Mothe BR, Weinfurter J, Wang C, Rehrauer W, Wilson N, Allen TM, Allison DB, Watkins DI (2003) Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 77:2736–2740
Mudd PA, Piaskowski SM, Neves PC, Rudersdorf R, Kolar HL, Eernisse CM, Weisgrau KL, de Santana MG, Wilson NA, Bonaldo MC, Galler R, Rakasz EG, Watkins DI (2010) The live-attenuated yellow fever vaccine 17D induces broad and potent T cell responses against several viral proteins in Indian rhesus macaques—implications for recombinant vaccine design. Immunogenetics 62:593–600
Muhl T, Krawczak M, Ten Haaft P, Hunsmann G, Sauermann U (2002) MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J Immunol 169:3438–3446
Nalca A, Livingston VA, Garza NL, Zumbrun EE, Frick OM, Chapman JL, Hartings JM (2010) Experimental infection of cynomolgus macaques (Macaca fascicularis) with aerosolized monkeypox virus. PLoS One 5(9):pii:e12880
Negri DR, Baroncelli S, Catone S, Comini A, Michelini Z, Maggiorella MT, Sernicola L, Crostarosa F, Belli R, Mancini MG, Farcomeni S, Fagrouch Z, Ciccozzi M, Boros S, Liljestrom P, Norley S, Heeney J, Titti F (2004) Protective efficacy of a multicomponent vector vaccine in cynomolgus monkeys after intrarectal simian immunodeficiency virus challenge. J Gen Virol 85:1191–1201
Otting N, Doxiadis GG, Bontrop RE (2009) Definition of Mafa-A and -B haplotypes in pedigreed cynomolgus macaques (Macaca fascicularis). Immunogenetics 61:745–753
Pendley CJ, Becker EA, Karl JA, Blasky AJ, Wiseman RW, Hughes AL, O'Connor SL, O'Connor DH (2008) MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics 60:339–351
Sauermann U, Siddiqui R, Suh YS, Platzer M, Leuchte N, Meyer H, Matz-Rensing K, Stoiber H, Nurnberg P, Hunsmann G, Stahl-Hennig C, Krawczak M (2008) Mhc class I haplotypes associated with survival time in simian immunodeficiency virus (SIV)-infected rhesus macaques. Genes Immun 9:69–80
Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD (2010) Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol 17:1170–1182
Weiss WR, Kumar A, Jiang G, Williams J, Bostick A, Conteh S, Fryauff D, Aguiar J, Singh M, O'Hagan DT, Ulmer JB, Richie TL (2007) Protection of rhesus monkeys by a DNA prime/poxvirus boost malaria vaccine depends on optimal DNA priming and inclusion of blood stage antigens. PLoS One 2:e1063
Wiseman RW, Karl JA, Bimber BN, O'Leary CE, Lank SM, Tuscher JJ, Detmer AM, Bouffard P, Levenkova N, Turcotte CL, Szekeres E Jr, Wright C, Harkins T, O'Connor DH (2009) Major histocompatibility complex genotyping with massively parallel pyrosequencing. Nat Med 15:1322–1326
Wiseman RW, Wojcechowskyj JA, Greene JM, Blasky AJ, Gopon T, Soma T, Friedrich TC, O'Connor SL, O'Connor DH (2007) Simian immunodeficiency virus SIVmac239 infection of major histocompatibility complex-identical cynomolgus macaques from Mauritius. J Virol 81:349–361
Acknowledgements
The authors thank the veterinary and support staff for their expertise and Julie-Ann Karl, Roger Wiseman and David O'Connor of the University of Wisconsin-Madison for their expert advice. This work was supported by the NIHR Centre for Research in Health Protection at the Health Protection Agency.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Microsatellite profiles of 90 Indonesian cynomolgus macaques. Previously described Mauritian haplotypes are denoted MCM 1, MCM 2, MCM 3, MCM 4, MCM 6 and coloured black, red, blue, green and grey, respectively. Novel Indonesian haplotypes are denoted IND 1–IND 30. RC recombinant haplotype; ND not determined: the haplotype could not be confirmed in two or more animals; 1–18 microsatellite markers previously described by Wiseman et al. (2007); P5, CA5-8 breeding group number; red text denotes animals assessed by RSCA as well as microsatellite analysis; asterisks denote animals where novel haplotypes were confirmed by analysis of one parental sample; double asterisks denote animals were novel haplotypes and were confirmed by analysis of both parental samples (DOCX 110 kb)
Rights and permissions
About this article
Cite this article
Mitchell, J.L., Mee, E.T., Almond, N.M. et al. Characterisation of MHC haplotypes in a breeding colony of Indonesian cynomolgus macaques reveals a high level of diversity. Immunogenetics 64, 123–129 (2012). https://doi.org/10.1007/s00251-011-0567-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-011-0567-z