Abstract
Background
Elevated fasting plasma glucose has been associated with increased risk for development of type 2 diabetes (T2D). The balance between glucokinase (GCK) and glucose-6-phosphate catalytic subunit 2 (G6PC2) activity are involved in glucose homeostasis through glycolytic flux, and subsequent insulin secretion.
Aim
In this study, we evaluated the association between the genetic variability of G6PC2 and GCK genes and T2D-related quantitative traits.
Methods
In 794 drug-naïve, GADA-negative, newly diagnosed T2D patients (VNDS; NTC01526720) we performed: genotyping of 6 independent tag-SNPs within GCK gene and 5 tag-SNPs within G6PC2 gene; euglycaemic insulin clamp to assess insulin sensitivity; OGTT to estimate beta-cell function (derivative and proportional control; DC, PC) by mathematical modeling. Genetic association analysis has been conducted using Plink software.
Results
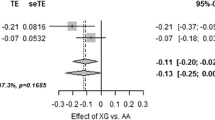
Two SNPs within GCK gene (rs882019 and rs1303722) were associated to DC in opposite way (both p < 0.004). Two G6PC2 variants (rs13387347 and rs560887) were associated to both parameters of insulin secretion (DC and PC) and to fasting C-peptide levels (all p < 0.038). Moreover, subjects carrying the A allele of rs560887 showed higher values of 2h-plasma glucose (2hPG) (p = 0.033). Haplotype analysis revealed that GCK (AACAAA) haplotype was associated to decreased fasting C-peptide levels, whereas, the most frequent haplotype of G6PC2 (GGAAG) was associated with higher fasting C-peptide levels (p = 0.001), higher PC (β = 6.87, p = 0.022) and the lower 2hPG (p = 0.012).
Conclusion
Our findings confirmed the role of GCK and G6PC2 in regulating the pulsatility in insulin secretion thereby influencing insulin-signaling and leading to a gradual modulation in glucose levels in Italian patients with newly diagnosed T2D.

Similar content being viewed by others
References
De Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CDA et al (2001) Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn study. J Am Med Assoc. https://doi.org/10.1001/jama.285.16.2109
Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T et al (2005) Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. https://doi.org/10.1056/NEJMoa050080
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU et al (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. https://doi.org/10.1038/ng.520
Bonadonna RC, Stumvoll M, Fritsche A, Muggeo M, Häring H, Bonora E et al (2003) Altered homeostatic adaptation of first- and second-phase β-cell secretion in the offspring of patients with type 2 diabetes: Studies with a minimal model to assess β-cell function. Diabetes. https://doi.org/10.2337/diabetes.52.2.470
Bouatia-Naji N, Rocheleau G, Van Lommel L et al (2008) A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 320(5879):1085–1088. https://doi.org/10.1126/science.1156849
Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, Sparsø T, Holmkvist J, Marchand M et al (2009) A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. https://doi.org/10.1038/ng.277
Vaxillaire M, Cavalcanti-Proença C, Dechaume A, Tichet J, Marre M, Balkau B et al (2008) The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes 57:2253–2257. https://doi.org/10.2337/db07-1807
Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB et al (2007) A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. https://doi.org/10.1038/ng2043
Hu C, Zhang R, Wang C, Ma X, Wang C, Fang Q et al (2009) A genetic variant of G6PC2 is associated with type 2 diabetes and fasting plasma glucose level in the Chinese population. Diabetologia. https://doi.org/10.1007/s00125-008-1241-3
Bianchi C, Dolci MA, Miccoli R, Cavalot F, Bonadonna RC, Cavallo GM et al (2012) Pathogenetic mechanisms and cardiovascular risk: differences between HbA1cand oral glucose tolerance test for the diagnosis of glucose tolerance. Diabetes Care. https://doi.org/10.2337/dc11-2504
Lehtovirta M, Kaprio J, Groop L, Trombetta M, Bonadonna RC (2005) Heritability of model-derived parameters of beta cell secretion during intravenous and oral glucose tolerance tests: a study of twins. Diabetologia. https://doi.org/10.1007/s00125-005-1815-2
Trombetta M, Bonetti S, Boselli ML et al (2013) PPARG2 Pro12Ala and ADAMTS9 rs4607103 as "insulin resistance loci" and "insulin secretion loci" in Italian individuals. The GENFIEV study and the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) 4. Acta Diabetol 50(3):401–408. https://doi.org/10.1007/s00592-012-0443-9
Walker M, Mari A, Jayapaul MK, Bennett SMA, Ferrannini E (2005) Impaired beta cell glucose sensitivity and whole-body insulin sensitivity as predictors of hyperglycaemia in non-diabetic subjects. Diabetologia. https://doi.org/10.1007/s00125-005-0004-7
Tura A, Grassi A, Winhofer Y, Guolo A, Pacini G, Mari A et al (2012) Progression to type 2 diabetes in women with former gestational diabetes: Time trajectories of metabolic parameters. PLoS ONE. https://doi.org/10.1371/journal.pone.0050419
Li H, Xu R, Peng X, Wang Y, Wang T (2013) Association of glucokinase regulatory protein polymorphism with type 2 diabetes and fasting plasma glucose: A meta-analysis. Mol Biol Rep. https://doi.org/10.1007/s11033-012-2470-6
Wall ML, Pound LD, Trenary I, O’Brien RM, Young JD (2015) Novel stable isotope analyses demonstrate significant rates of glucose cycling in mouse pancreatic islets. Diabetes. https://doi.org/10.2337/db14-0745
Wang Y, Martin CC, Oeser JK, Sarkar S, McGuinness OP, Hutton JC et al (2007) Deletion of the gene encoding the islet-specific glucose-6-phosphatase catalytic subunit-related protein autoantigen results in a mild metabolic phenotype. Diabetologia. https://doi.org/10.1007/s00125-006-0564-1
Pound LD, Oeser JK, O’Brien TP, Wang Y, Faulman CJ, Dadi PK et al (2013) G6PC2: a negative regulator of basal glucose-stimulated insulin secretion. Diabetes. https://doi.org/10.2337/db12-1067
Bosma KJ, Rahim M, Singh K, Goleva SB, Wall ML, Xia J et al (2020) Pancreatic islet beta cell-specific deletion of G6pc2 reduces fasting blood glucose. J Mol Endocrinol. https://doi.org/10.1530/JME-20-0031
Westermeier F, Holyoak T, Asenjo JL, Gatica R, Nualart F, Burbulis I et al (2019) Gluconeogenic enzymes in β-cells: pharmacological targets for improving insulin secretion. Trends Endocrinol Metab. https://doi.org/10.1016/j.tem.2019.05.004
Al-Daghri NM, Pontremoli C, Cagliani R, Forni D, Alokail MS, Al-Attas OS et al (2017) Susceptibility to type 2 diabetes may be modulated by haplotypes in G6PC2, a target of positive selection. BMC Evol Biol. https://doi.org/10.1186/s12862-017-0897-z
Mahajan A, Sim X, Ng HJ, Manning A, Rivas MA, Highland HM et al (2015) Identification and functional characterization of G6PC2 coding variants influencing glycemic traits define an effector transcript at the G6PC2-ABCB11 locus. PLoS Genet. https://doi.org/10.1371/journal.pgen.1004876
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 42(Suppl 1):S13–S28. doi: https://doi.org/10.2337/dc19-S002. PMID: 30559228.
Bonetti S, Trombetta M, Malerba G, Boselli L, Trabetti E, Muggeo M et al (2011) Variants and haplotypes of TCF7L2 are Associated with β-cell function in patients with newly diagnosed type 2 diabetes: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) 1. J Clin Endocrinol Metab 96:E389–E393. https://doi.org/10.1210/jc.2010-1677
Bonora E, Trombetta M, Dauriz M, Travia D, Cacciatori V, Brangani C et al (2020) Chronic complications in patients with newly diagnosed type 2 diabetes: prevalence and related metabolic and clinical features: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) 9. BMJ Open Diabetes Res Care 8:e001549. https://doi.org/10.1136/bmjdrc-2020-001549
Bonetti S, Trombetta M, Boselli ML, Turrini F, Malerba G, Trabetti E et al (2011) Variants of GCKR affect both β-cell and kidney function in patients with newly diagnosed type 2 diabetes: the Verona newly diagnosed type 2 diabetes study 2. Diabetes Care 34:1205–1210. https://doi.org/10.2337/dc10-2218
Bonadonna RC, Heise T, Arbet-Engels C, Kapitza C, Avogaro A, Grimsby J et al (2010) Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab 95:5028–5036. https://doi.org/10.1210/jc.2010-1041
Lin CH, Yeakley JM, McDaniel TK, Shen R (2009) Medium- to high-throughput SNP genotyping using VeraCode microbeads. Methods Mol Biol. https://doi.org/10.1007/978-1-59745-553-4_10
Purcell S, Neale B, Todd-brown K, Thomas L, Ferreira MAR, Bender D et al (2007) REPORT PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. https://doi.org/10.1086/519795
Kolb H, Martin S (2017) Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. https://doi.org/10.1186/s12916-017-0901-x
Kautzky-Willer A, Harreiter J, Pacini G (2016) Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. https://doi.org/10.1210/er.2015-1137
Chen WM, Erdos MR, Jackson AU, Saxena R, Sanna S, Silver KD et al (2008) Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest. https://doi.org/10.1172/JC134566
Reiling E, Van ’t Riet E, Groenewoud MJ, Welschen LMC, Van Hove EC, Nijpels G et al (2009) Combined effects of single-nucleotide polymorphisms in GCK, GCKR, G6PC2 and MTNR1B on fasting plasma glucose and type 2 diabetes risk. Diabetologia 52(2):1866–1870. https://doi.org/10.1007/s00125-009-1413-9
Gómez-Zumaquero JM, Rojo-Martínez G, García-Escobar E, Martín-Nũez GM, Haro J, Esteva I et al (2008) The -30G>A polymorphism of the glucokinase gene promoter is associated with obesity in a population from southern Spain. Obesity. https://doi.org/10.1038/oby.2008.265
Stone LM, Kahn SE, Fujimoto WY, Deeb SS, Porte D Jr (1996) A variation at position -30 of the beta-cell glucokinase gene promoter is associated with reduced beta-cell function in middle-aged Japanese-American men. Diabetes 45(4):422–428. https://doi.org/10.2337/diab.45.4.422
Urhammer SA, Hansen T, Clausen JO, Eiberg H, Pedersen O (1998) The g/a nucleotide variant at position -30 in the β-cell-specific glucokinase gene promoter has no impact on the β-cell function in Danish caucasians. Diabetes. https://doi.org/10.2337/diab.47.8.1359
Hu C, Zhang R, Wang C, Yu W, Lu J, Ma X et al (2010) Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS ONE. https://doi.org/10.1371/journal.pone.0011761
Boortz KA, Syring KE, Pound LD, Wang Y, Oeser JK, O’Brien RM (2016) Functional analysis of mouse G6pc1 mutations using a novel in situ assay for glucose-6-phosphatase activity and the effect of mutations in conserved human G6PC1/G6PC2 amino acids on G6PC2 protein expression. PLoS ONE. https://doi.org/10.1371/journal.pone.0162439
Shi Y, Li Y, Wang J, Wang C, Fan J, Zhao J et al (2017) Meta-analyses of the association of G6PC2 allele variants with elevated fasting glucose and type 2 diabetes. PLoS ONE. https://doi.org/10.1371/journal.pone.0181232
Li X, Shu YH, Xiang AH, Trigo E, Kuusisto J, Hartiala J et al (2009) Additive effects of genetic variation in GCK and G6PC2 on insulin secretion and fasting glucose. Diabetes. https://doi.org/10.2337/db09-0228
Heni M, Ketterer C, Hart LM et al (2010) The impact of genetic variation in the G6PC2 gene on insulin secretion depends on glycemia. J Clin Endocrinol Metab 95(12):E479–E484. https://doi.org/10.1210/jc.2010-0860
Arden SD, Zahn T, Steegers S, Webb S, Bergman B, O’Brien RM et al (1999) Molecular cloning of a pancreatic islet-specific glucose-6-phosphatase catalytic subunit-related protein. Diabetes. https://doi.org/10.2337/diabetes.48.3.531
O’Brien RM (2013) Moving on from GWAS: Functional studies on the G6PC2 gene implicated in the regulation of fasting blood glucose. Curr Diab Rep. https://doi.org/10.1007/s11892-013-0422-8
Warner JP, Leek JP, Intody S, Markham AF, Bonthron DT (1995) Human glucokinase regulatory protein (GCKR): cDNA and genomic cloning, complete primary structure, and chromosomal localization. Mamm Genome. https://doi.org/10.1007/BF00356171
Acknowledgment
The technical help of Monica Zardini and Federica Moschetta (Department of Medicine, University of Verona, Italy) is gratefully acknowledged.
Funding
This study was supported in part by a European Foundation for the Study of Diabetes/Novartis grant (to RCB) and by research grants of University of Verona (to RCB and EB).
Author information
Authors and Affiliations
Contributions
CZ and ER researched and analysed data and cowrote the manuscript. SB genotyped all samples and discussed the article. MLB carried out mathematical modelling of the data. ET and GM discussed the article. RCB developed the mathematical models and designed the study. EB edited the manuscript and provided substantial contribution to the overall manuscript. MT is the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Research involving human participants and/or animals
We studied the genetics of a cohort and in the main text is reported that the research was approved by the Human Investigation Committee of the Verona City Hospital and the study was conducted in accordance with the Declaration of Helsinki.
Informed consent
Written consent was obtained from all study participants after a full explanation of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
C. Zusi and E. Rinaldi these authors contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zusi, C., Rinaldi, E., Bonetti, S. et al. Haplotypes of the genes (GCK and G6PC2) underlying the glucose/glucose-6-phosphate cycle are associated with pancreatic beta cell glucose sensitivity in patients with newly diagnosed type 2 diabetes from the VNDS study (VNDS 11). J Endocrinol Invest 44, 2567–2574 (2021). https://doi.org/10.1007/s40618-020-01483-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01483-3