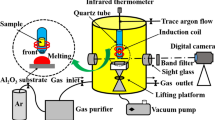
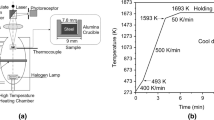
Abstract
Behavior of inclusions as particles on liquid Ultra-Low C (ULC) steel was investigated both by an in-situ observation and a theoretical analysis. The behavior was examined in view of Ti content in the steel as an alloying component and of oxygen potential exerted on the surface of the steel melt. A confocal scanning laser microscopy with a gold image furnace was used for the observation, and the force exerted between two particles was extracted. It was found that the inclusions showed attraction each other, and agglomerated. When the oxygen potential was low (\(P_{\mathrm{O}_2} \simeq 10^{-22}\) bar), the presence of Ti (\([\%\ \text{Ti}] = 0.0735\)) did not influence on the agglomeration force as well as the acceleration. However, increasing \(P_{\mathrm{O}_2}\) (\(\simeq 10^{-15}\) bar) resulted in slight decrease of the acceleration. When Ti content was very low (\([\%\ \text{Ti}] =0.0018\)), \(P_{\mathrm{O}_2}\) did not influence the attraction. A post-mortem analysis of the inclusion composition revealed that the inclusions on the Ti-free steel surface were mostly alumina regardless of the \(P_{\mathrm{O}_2}\) employed in the present study, while those on the Ti-added steel were composed of \(\text{Fe}_t\text{O}\)–\(\text{Al}_2\text{O}_3\) (low \(P_{\mathrm{O}_2}\)) or \(\text{Fe}_t\text{O}\)–\(\text{TiO}_x\)(–\(\text{Al}_2\text{O}_3\)) (high \(P_{\mathrm{O}_2}\)). From the analysis using Kralchevski-Paunov model for lateral capillary force between two spherical particles, it is suggested that the formation of \(\text{Fe}_t\text{O}\)-containing oxidation product lowers the contact angle between the inclusion and the liquid steel, thereby lowering the agglomeration force as well as the acceleration. However, the model generally underestimated the agglomeration force.
Graphical abstract











Similar content being viewed by others
Notes
In addition to this, if the \(\text{Fe}_t\text{O}\) containing liquid oxide forms between the inclusion and the liquid steel, the \(\alpha _k\) in the model would mean the contact angle between the inclusion and the liquid oxide. In such case, an interpretation of the capillary model is different to the previous cases.
For a general case in the present study, \(\rho _{\mathrm{I}} = 7000\ \text{kg}\ \text{m}^{-3}\), \(\rho _{\mathrm{II}} = 0\ \text{kg}\ \text{m}^{-3}\), \(g = 9.8\ \text{m}\ \text{s}^{-2}\), \(\gamma = 1.4\ \text{N}\ \text{m}^{-1}\), \(R_k =10^{-5}\ \text{m}\) yields \((qR_k)^2 = 5 \times 10^{-6}\), satisfying the above condition.
The summation for \(h_k\) and A rapidly decays. In the present study, the summation continued up to \(n =10\).
References
J.R. Fekete, D.C. Strugala, Z. Yao, JOM 44, 17 (1992). https://doi.org/10.1007/BF03222745
J.-I. Jang, Y. Kang, Met. Mater. Int. 27, 4814 (2021). https://doi.org/10.1007/s12540-020-00696-8
N. Fukuda, Tetsu-to-Hagane 59, 231 (1973). https://doi.org/10.2355/tetsutohagane1955.59.2_231
Y. Hiraga, Y. Yashima, K. Fujii, Taikabutsu Overseas 15, 22 (1995)
S. Basu, S.K. Choudhary, N.U. Girase, ISIJ Int. 44, 1653–1660 (2004)
H. Cui, Y.-P. Bao, M. Wang, W.-S. Wu, Int. J. Min. Met. Mater. 17, 154 (2010). https://doi.org/10.1007/s12613-010-0206-y
J.-H. Lee, M.-H. Kang, S.-K. Kim, J. Kim, M.-S. Kim, Y.-B. Kang, ISIJ Int. 59, 749 (2019). https://doi.org/10.2355/isijinternational.ISIJINT-2018-672
H. Tsunekawa, T. Yamashita, T. Aoyama, R. Sugihara, Tetsu-to-Hagane 102, 202 (2016). https://doi.org/10.2355/tetsutohagane.TETSU-2015-061
Y.-B. Kang, H.-G. Lee, ISIJ Int. 50, 501 (2010). https://doi.org/10.2355/isijinternational.50.501
K. Seo, K.-H. Kim, H.J. Kim, H. Ryoo, G.M. Evans, C. Lee, Met. Mater. Int. 26, 1226 (2020). https://doi.org/10.1007/s12540-019-00390-4
J.-H. Sim, T.-Y. Kim, J.-Y. Kim, C.-W. Kim, J.-H. Chung, J. Moon, C.-H. Lee, H.-U. Hong, Met. Mater. Int. 28, 337 (2022). https://doi.org/10.1007/s12540-020-00870-y
S. Ghosh, M.C. Somani, D. Setman, S. Mula, Met. Mater. Int. 27, 2481 (2021). https://doi.org/10.1007/s12540-020-00827-1
B. Wang, J. Li, Met. Mater. Int. 27, 2656 (2021). https://doi.org/10.1007/s12540-020-00617-9
H. Matsuura, C. Wang, G. Wen, S. Sridhar, ISIJ Int. 47, 1265 (2007). https://doi.org/10.2355/isijinternational.47.1265
C. Wang, N.T. Nuhfer, S. Sridhar, Metall. Mater. Trans. B 40, 1022 (2009). https://doi.org/10.1007/s11663-009-9290-7
C. Wang, N. Nuhfer, S. Sridhar, Metall. Mater. Trans. B 40, 1005 (2009). https://doi.org/10.1007/s11663-009-9267-6
C. Wang, N.T. Nuhfer, S. Sridhar, Metall. Mater. Trans. B 41, 1084 (2010). https://doi.org/10.1007/s11663-010-9397-x
C. Wang, N. Verma, Y. Kwon, W. Tiekink, N. Kikuchi, S. Sridhar, ISIJ Int. 51, 375 (2011). https://doi.org/10.2355/isijinternational.51.375
M.-A.V. Ende, M. Guo, R. Dekkers, M. Burty, J.V. Dyck, P.T. Jones, B. Blanpain, P. Wollants, ISIJ Int. 49, 1133 (2009). https://doi.org/10.2355/isijinternational.49.1133
C. Bernhard, P. Dorrer, S. Michelic, R. Rössler, BHM Berg- und Hüttenmännische Monatshefte 164, 475 (2019). https://doi.org/10.1007/s00501-019-00900-2
P. Dorrer, S.K. Michelic, C. Bernhard, A. Penz, R. Rössler, Steel Res. Int. 90, 1800635 (2019). https://doi.org/10.1002/srin.201800635
W.-C. Doo, D.-Y. Kim, S.-C. Kang, K.-W. Yi, Met. Mater. Int. 13, 249 (2007). https://doi.org/10.1007/BF03027813
M.-K. Sun, I.-H. Jung, H.-G. Lee, Met. Mater. Int. 14, 791 (2008). https://doi.org/10.3365/met.mat.2008.12.791
K. Das, P. Choudhury, S. Das, J. Phase Equi. 23, 525 (2002)
Y.-J. Park, W.-Y. Kim, Y.-B. Kang, J. Eur. Ceram. Soc. 41, 7362 (2021). https://doi.org/10.1016/j.jeurceramsoc.2021.06.052
J.-H. Lee, M.-H. Kang, S.-K. Kim, Y.-B. Kang, ISIJ Int. 58, 1257 (2018). https://doi.org/10.2355/isijinternational.ISIJINT-2018-164
J.-H. Lee, S.-K. Kim, M.-H. Kang, Y.-B. Kang, BHM Berg- und Hüttenmännische Monatshefte 163, 18 (2018). https://doi.org/10.1007/s00501-017-0687-3
K. Sasai, Y. Mizukami, ISIJ Int. 41, 1331 (2001). https://doi.org/10.2355/isijinternational.41.1331
T. Mizoguchi, Y. Ueshima, M. Sugiyama, K. Mizukami, ISIJ Int. 53, 639 (2013). https://doi.org/10.2355/isijinternational.53.639
J.-H. Lee, Y.-B. Kang, ISIJ Int. 60, 426 (2020). https://doi.org/10.2355/isijinternational.ISIJINT-2019-384
H. Yin, H. Shibata, T. Emi, M. Suzuki, ISIJ Int. 37, 946 (1997). https://doi.org/10.2355/isijinternational.37.946
H. Yin, H. Shibata, T. Emi, M. Suzuki, ISIJ Int. 37, 936 (1997). https://doi.org/10.2355/isijinternational.37.936
S. Kimura, K. Nakajima, S. Mizoguchi, Metall. Mater. Trans. B 32, 79 (2001). https://doi.org/10.1007/s11663-001-0010-1
S. Vantilt, B. Coletti, B. Blanpain, J. Fransaer, P. Wollants, S. Sridhar, ISIJ Int. 44, 1 (2004). https://doi.org/10.2355/isijinternational.44.1
J. Wikström, K. Nakajima, H. Shibata, A. Tilliander, P. Jönsson, Mater. Sci. Eng. A 495, 316 (2008). https://doi.org/10.1016/j.msea.2007.09.084
J. Appelberg, K. Nakajima, H. Shibata, A. Tilliander, P. Jönsson, Mater. Sci. Eng. A 495, 330 (2008). https://doi.org/10.1016/j.msea.2007.12.051
Y. Kang, B. Sahebkar, P.R. Scheller, K. Morita, D. Sichen, Metall. Mater. Trans. B 42, 522 (2011). https://doi.org/10.1007/s11663-011-9497-2
C. Xuan, A.V. Karasev, P.G. Jönsson, K. Nakajima, Steel Res. Int. 88, 1600090 (2017). https://doi.org/10.1002/srin.201600090
S.K. Michelic, U.D. Salgado, C. Bernhard, IOP Conf. Ser. Mater. Sci. Eng. 143, 012010 (2016). https://doi.org/10.1088/1757-899X/143/1/012010
G. Du, J. Li, Z.-B. Wang, C.-B. Shi, Steel Res. Int. 88, 1600185 (2017). https://doi.org/10.1002/srin.201600185
W. Mu, N. Dogan, K.S. Coley, Metall. Mater. Trans. B 48, 2092 (2017). https://doi.org/10.1007/s11663-017-0998-5
W. Mu, N. Dogan, K.S. Coley, Metall. Mater. Trans. B 48, 2379 (2017). https://doi.org/10.1007/s11663-017-1027-4
B. Coletti, B. Blanpain, S. Vantilt, S. Sridhar, Metall. Mater. Trans. B 34, 533 (2003). https://doi.org/10.1007/s11663-003-0021-1
W. Mu, N. Dogan, K.S. Coley, JOM 70, 1199 (2018). https://doi.org/10.1007/s11837-018-2893-1
L. Cao, G. Wang, Y. Zhao, S. Sridhar, H. Lei, Metall. Mater. Trans. B 50, 2502 (2019). https://doi.org/10.1007/s11663-019-01688-9
C. Xuan, W. Mu, J. Mater. Sci. 54, 8684 (2019). https://doi.org/10.1007/s10853-019-03458-z
W. Mu, C. Xuan, Metall. Mater. Trans. B 50, 2694 (2019). https://doi.org/10.1007/s11663-019-01686-x
G. Wang, Y. Zhao, Y. Xiao, P. Jin, S. Li, S. Sridhar, Metall. Mater. Trans. B 51, 3051 (2020). https://doi.org/10.1007/s11663-020-01978-7
Y. Wang, C. Liu, Metall. Mater. Trans. B 51, 2585 (2020). https://doi.org/10.1007/s11663-020-01938-1
L. Wang, S. Yang, J. Li, C. Chen, C. Li, X. Li, Steel Res. Int. 97, 2100013 (2021). https://doi.org/10.1002/srin.202100013
Y. Wang, C. Liu, ISIJ Int. 61, 1396 (2021). https://doi.org/10.2355/isijinternational.ISIJINT-2020-684
Y. Li, L. Wang, J. Li, S. Yang, C. Chen, C. Li, X. Li, ISIJ Int. 61, 753 (2021). https://doi.org/10.2355/isijinternational.ISIJINT-2020-585
K. Nakajima, S. Mizoguchi, Metall. Mater. Trans. B 32, 629 (2001). https://doi.org/10.1007/s11663-001-0118-3
K. Sasai, ISIJ Int. 54, 2780 (2014). https://doi.org/10.2355/isijinternational.54.2780
K. Sasai, ISIJ Int. 56, 1013 (2016). https://doi.org/10.2355/isijinternational.ISIJINT-2016-038
K. Sasai, ISIJ Int. 58, 469 (2018). https://doi.org/10.2355/isijinternational.ISIJINT-2017-480
K. Sasai, ISIJ Int. 60, 409 (2020). https://doi.org/10.2355/isijinternational.ISIJINT-2019-107
L. Zheng, A. Malfliet, P. Wollants, B. Blanpain, M. Guo, ISIJ Int. 55, 1891 (2015). https://doi.org/10.2355/isijinternational.ISIJINT-2015-099
L. Zheng, A. Malfliet, P. Wollants, B. Blanpain, M. Guo, ISIJ Int. 56, 926 (2016). https://doi.org/10.2355/isijinternational.ISIJINT-2015-561
L. Zheng, A. Malfliet, P. Wollants, B. Blanpain, M. Guo, ISIJ Int. 56, 1529 (2016). https://doi.org/10.2355/isijinternational.ISIJINT-2015-621
L. Zheng, A. Malfliet, P. Wollants, B. Blanpain, M. Guo, Acta Mater. 120, 443 (2016). https://doi.org/10.1016/j.actamat.2016.08.046
P. Presoly, R. Pierer, C. Bernhard, Metall. Mater. Trans. A 44, 5377 (2013). https://doi.org/10.1007/s11661-013-1671-5
Y. M. Cho, Y.-J. Park, D.-H. Kim, M.-H. Song, Y.-B. Kang, Inclusion evolution on surface of liquid ultra low carbon steel: CSLM Investigation and composition analysis, Paper presented at 2021 Spring Conference of the Korean Institute of Metals and Materials, Wellihillipark, Hoengseong, pp. 28–30 (2021)
J.-H. Lee, Y.-B. Kang, ISIJ Int. 60, 258 (2020). https://doi.org/10.2355/isijinternational.ISIJINT-2019-355
C. Bale, E. Bélisle, P. Chartrand, S. Decterov, G. Eriksson, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A. Pelton, C. Robelin, S. Petersen, Calphad 33, 295 (2009). https://doi.org/10.1016/j.calphad.2008.09.009
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, M.-A. Van Ende, Calphad 54, 35 (2016). https://doi.org/10.1016/j.calphad.2016.05.002
Image J, developed at U.S. National Institutes of Health. http://imagej.nih.gov/ij/
B.J. Monaghan, L. Chen, J. Non-Crystall. Solids 347, 254 (2004). https://doi.org/10.1016/j.jnoncrysol.2004.09.011
P. Kralchevsky, V. Paunov, I. Ivanov, K. Nagayama, J. Colloid Int. Sci. 151, 79 (1992). https://doi.org/10.1016/0021-9797(92)90239-I
P.A. Kralchevsky, V.N. Paunov, N.D. Denkov, I.B. Ivanov, K. Nagayama, J. Colloid Int. Sci. 155, 420 (1993)
P.A. Kralchevsky, N.D. Denkov, V.N. Paunov, O.D. Velev, I.B. Ivanov, H. Yoshimura, K. Nagayama, J. Physics, Condens. Matter 6, A395 (1994). https://doi.org/10.1088/0953-8984/6/23A/065
V.N. Paunov, P.A. Kralchevsky, N.D. Denkov, K. Nagayama, J. Colloid Int. Sci. 157, 100 (1993). https://doi.org/10.1006/jcis.1993.1163
K. Nogi, K. Ogino, Can. Metall. Quart. 22, 19 (1983). https://doi.org/10.1179/cmq.1983.22.1.19
A. Karasangabo, C. Bernhard, J. Adhes. Sci. Technol. 26, 1141 (2012). https://doi.org/10.1163/016942411X580252
K. Nakashima, K. Takihira, K. Mori, N. Shinozaki, J. Jpn. Inst. Met. 55, 1199 (1991). https://doi.org/10.2320/jinstmet1952.55.11_1199
N. Takiuchi, T. Taniguchi, N. Shinozaki, K. Mukai, J. Jpn. Inst. Met. 55, 44 (1991). https://doi.org/10.2320/jinstmet1952.55.1_44
N. Takiuchi, T. Taniguchi, Y. Tanaka, N. Shinozaki, K. Mukai, J. Jpn. Inst. Met. 55, 180 (1991). https://doi.org/10.2320/jinstmet1952.55.2_180
K. Ogino, K. Nogi, Y. Koshida, Tetsu-to-Hagané 59, 1380 (1973). https://doi.org/10.2355/tetsutohagane1955.59.10_1380
H. Barati, M. Wu, S. Michelic, S. Ilie, A. Kharicha, A. Ludwig, Y-B. Kang, H. Barati, M.S. Wu, Metall. Mater. Trans. B 52B, 4167 (2021). https://doi.org/10.1007/s11663-021-02336-x
D. Chan, J. Henry, L. White, J. Colloid Int. Sci. 79, 410 (1981). https://doi.org/10.1016/0021-9797(81)90092-8
B.V. Derjaguin, Dokl. Akad. Nauk USSR 51, 517 (1946)
Acknowledgements
This research was financially supported by POSCO, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Kralchevski–Paunov Model
Appendix: Kralchevski–Paunov Model
Capillary force between two floating particles on a fluid phase (I) with higher density (\(\rho _{\mathrm{I}}\)) which is immiscible with the other fluid phase (II) with lower density (\(\rho _{\mathrm{II}}\)) was formulated by Kralchevsky et al. [69,70,71,72]. In the present study, formulation given by Paunov et al. was employed [72].
Figure 11 shows four different cases of floating particle between phase I and phase II. The particle k was assumed to be a sphere with a radius \(R_k\). In the case of inclusion floating on liquid steel surface, the phases I and II correspond to liquid steel and gas, respectively. Figure 11a and b show cases when the particle wets to the phase II, while Fig. 11c and d show other cases when the particle does not wet to the phase II. Therefore, the contact angle (\(\alpha _k\)) is lower than \(90^\circ\) ((a) and (b)), or is higher than 90 \(^\circ \) ((c) and (d)). Figure 11a and c correspond to cases of lighter particle, therefore, center of the particle is located above the horizontal line separating the phase I and the phase II (\(Z_c^k > 0\)). Figure 11b and d refer to other cases of heavier particle (\(Z_c^k < 0\)). Meniscus is shown by a red curve for each case (see online version for the color). Characteristic geometric variables can be seen in the figure (\(r_k\): radius of the contact line, \(h_k\): the meniscus elevation, \(\psi _k\): slope at the respective contact line, \(b_k\): depth of immersion).
When two particles (\(k=1\) and 2) approach each other, the above geometric variables vary due to suppression/elevation of the meniscus between the two particles. The energy change (\(\Delta W\)) due to the change of the distance between the two particles consists of three parts: gravitational, wetting, and meniscus contributions. After taking into account the three contributions to the total energy along with the geometric relationship, Paunov et al. derived the following equation [72]:
where g, \(\gamma \), \(V_s^k\), \(V_l^k\), and \(W_\infty \) are the gravitational acceleration (in \(\text{m}\ \text{s}^{-2}\)), the surface tension of the phase I (if the phase II is gas) (in \(\text{N}\ \text{m}^{-1}\)), the volume of the particle k (in \(\text{m}^3\)), the volume of the part of the particle which is immersed into the phase I (in \(\text{m}^3\)), and the energy of the system when the two particles are separated infinitely, respectively. \(D_k\) is the density ratio:
Details of the derivation is out of scope of the present study, and the readers are invited to read [72].
The lateral capillary force in the limit of \((qR_k)^2<< 1\) is obtained byFootnote 3:
where L and \(Q_k\) are the distance between centers of two particles (in m) and the capillary charge (\(= r_k \sin \psi _k\), in m), and q are the reciprocal of capillary length (\(\text{m}^{-1}\)):
The \(Q_k\) is introduced because the vertical force due to the meniscus surface tension \(\gamma \) is counterbalanced by the gravitational force (\(F_g^k\)):
In the limit of \((qL)^2<< 1\), the Eq. (14) is reduced to:
In order to calculate the capillary lateral force F, it is necessary to calculate \(Q_k\) at each L. The following procedure is taken from Paunov et al. [72]. When the two particles are separated by a long distance, e.g. \(L = \infty \), Chan et al. derived the following expression for the \(Q_k\) [80]:
They also presumed that the meniscus slope is small under the condition. Paunov et al. interpreted this as \(\cos \psi _\infty \approx 1\), and the following equation for \(r_k\) was derived from the geometry of this system (Fig. 11):
Using the Eqs. (16), (17), (20), \(r_{k,\infty }\) was derived:
The elevation of the contact line at \(L = \infty \) (\(h_{k,\infty }\)) was independently reported by Derjaquin as [81]:
where \(\gamma _e = 1.781072418\cdots \) (\(\ln \gamma _e\) is the Euler-Mascheroni constant). From the geometry of this system (Fig. 11), the immersion depth \(b_{k,\infty }\) is obtained as:
Using the known physicochemical properties (\(\rho _{\mathrm{I}}\), \(\rho _{\mathrm{II}}\), \(\rho _k\), \(\alpha _k\), and \(\gamma \)), the above four parameters (\(Q_{k,\infty }\), \(r_{k,\infty }\), \(h_{k,\infty }\), and \(b_{k,\infty }\)) were obtained for the particle k (\(\text{radius}=R_k\), \(k=1\) and 2) at \(L = \infty \).
\(Q_k\), \(r_k\), \(h_k\), and \(b_k\) vary as L varies via changing \(\psi _k\), because of change of the meniscus. This generates a lateral force between the two particles. Kralchevsky et al. [70] proposed the following general expression for \(h_k\) in the limit of \((qL)^2<< 1\):
where
respectivelyFootnote 4
From force balance and geometry of the system, a general equation for \(Q_k\) is derived as:
The radius of the contact line (\(r_k\)) can be also obtained from the geometry of the system by somewhat different way used in the Eq. (20):
Using the Eqs. (16), (17), \(\psi _k\) is obtained as:
and \(b_k\) in general condition is written as:
which is essentially the same as the Eq. (23).
The capillary lateral force is then calculated as follows.
-
1.
Set the particle radius \(R_1\) and \(R_2\).
-
2.
Calculate \(D_1\), \(D_2\), and q from all available the physicochemical properties \(\gamma \), \(\rho _{\mathrm{I}}\), \(\rho _{\mathrm{II}}\), \(\rho _1\) and \(\rho _2\).
-
3.
Calculate \(Q_{k,\infty }\), \(r_{k,\infty }\), \(h_{k,\infty }\), and \(b_{k,\infty }\) using the Eqs. (19), (21), (22), and (23), respectively.
-
4.
Set \(Q_k = Q_{k,\infty }\), \(r_k = r_{k,\infty }\), \(h_k =h_{k,\infty }\), \(b_k = b_{k,\infty }\), and \(\psi _k =\psi _{k,\infty }\).
-
5.
\(h_k\) at a finte L is obtained by the Eq. (24) along with the Eqs. (25) to (27), the updated \(Q_k\) and \(r_k\).
-
6.
\(Q_k\), \(r_k\), \(\psi _k\), and \(b_k\) are obtained by the Eqs. (28), (29), (30), and (31), in the order, respectively.
-
7.
\(h_k\) at the L is calculated by the Eq. (24) along with the Eqs. (25) to (27), followed by the calculations for \(Q_k\), \(r_k\), \(\psi _k\), and \(b_k\) by the Eqs. (28), (29), (30), and (31), respectively. This is repeated until all the calculated values are converged after this iteration.
-
8.
F at the L is calculated using the Eq. (18).
Rights and permissions
About this article
Cite this article
Kim, DH., Choi, JB., Hong, HM. et al. Inclusion Agglomeration on Ultra-Low C Liquid Steel Surface: Roles of Ti in the Steel and the Oxygen Potential. Met. Mater. Int. 28, 3106–3119 (2022). https://doi.org/10.1007/s12540-022-01190-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-022-01190-z