Abstract
Genital morphology in animals with internal fertilization is considered to be among the fastest evolving traits. Sexual selection is often proposed as the main driver of genital diversification but the exact selection mechanisms involved are usually unclear. In addition, the mechanisms operating may differ even between pairs of sibling species. We investigated patterns of male genital variation within and between natural populations of the cactophilic fly Drosophila koepferae ranging its entire geographic distribution and compared them with those previously observed in its sibling species, D. buzzatii. Using both mtDNA and nDNA markers we found that genital shape variation in D. koepferae is more restricted than expected for neutral evolution, suggesting the predominance of stabilizing selection. We also detected dissimilar patterns of divergence between populations of D. koepferae that were allopatric and sympatric with D. buzzatii. The constrained evolution inferred for D. koepferae’s genitalia clearly contrasts with the rapid divergence and higher morphological disparity observed in the populations of D. buzzatii. Finally, different possible scenarios of male genital evolution in each species and within the radiation of D. buzzatii cluster are discussed.

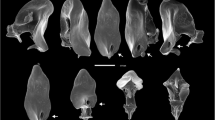
(modified from Soto et al. 2007); b aedeagus of the seven recognized species of the Drosophila buzzatii cluster





Similar content being viewed by others
References
Anderson, C. M., & Langerhans, R. B. (2015). Origins of female genital diversity: Predation risk and lock-and-key explain rapid divergence during an adaptive radiation. Evolution, 69, 2452–2467.
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26, 32–46.
Arnqvist, G. (1997). The evolution of animal genitalia: Distinguishing between hypotheses by single species studies. Biological Journal of the Linnean Society, 60, 365–379.
Arnqvist, G. (1998). Comparative evidence for the evolution of genitalia by sexual selection. Nature, 393(6687), 784–786.
Bonhomme, V., Picq, S., Gaucherel, C., & Claude, J. (2014). Momocs: Outline analysis using R. Journal of Statistical Software, 56, 1–24.
Brennan, P. L. R., & Prum, R. O. (2015). Mechanism and evidence of genital coevolution: The roles of natural selection, mate choice, and sexual conflict. Cold Spring Harbor Perspectives in Biology, 7, a017749.
Brommer, J. E. (2011). Whither PST? On the approximation of QST by PST in conservation and evolutionary biology. Journal of Evolutionary Biology, 24, 1160–1168.
Carreira, V. P., Soto, I. M., Hasson, E., & Fanara, J. J. (2006). Patterns of variation in wing morphology in the cactophilic Drosophila buzzatii and its sibling D. koepferae. Journal of Evolutionary Biology, 9, 1275–1282.
Cordero, C., & Eberhard, W. G. (2005). Interaction between sexually antagonistic selection and mate choice in the evolution of female responses to male traits. Evolutionary Ecology, 19, 111–122.
De Panis, D., Padró, J., Furió-Tarí, P., Tarazona, S., Soto, I. M., Carmona, M., Conesa, P. A., & Hasson, E. (2016). Transcriptome modulation during host shift is driven by secondary metabolites in desert Drosophila. Molecular Ecology, 25(18), 4534–4550.
Dommergues, C. H., Dommergues, J. L., & Verrecchia, E. P. (2007). The discrete cosine transform, a Fourier-related method for morphometric analysis of open contours. Mathematical Geology, 39, 749–763.
Dufour, L. (1844). Anatomie générale des Diptères. Annales des Sciences Naturelles, 1, 244–264.
Eberhard, W. G. (1985). Sexual selection and animal genitalia. Cambridge: Harvard University Press.
Eberhard, W. G. (1993). Evaluating models of sexual selection: Genitalia as a test case. The American Naturalist, 142, 564–571.
Eberhard, W. G. (2010). Evolution of genitalia: Theories, evidence, and new directions. Genetica, 138, 5–18.
Excoffier, L. (2009). Arlequin Ver 3.5. An integrated software package for population genetics data analysis. Bern: Swiss Institute of Bioinformatics, Universitat Bern.
Fanara, J. J., Folguera, G., Iriarte, P. F., Mensch, J., & Hasson, E. (2006). Genotype by environment interactions in viability and developmental time in populations of cactophilic Drosophila. Journal of Evolutionary Biology, 19, 900–908.
Fanara, J. J., Fontdevila, A., & Hasson, E. (1999). Oviposition preference and life history traits in cactophilic Drosophila koepferae and D. buzzatii in association with their natural hosts. Evolutionary Ecology, 13, 173–190.
Foote, M. (1993). Contributions of individual taxa to overall morphological disparity. Paleobiology, 19, 403–419.
Foote, M. (1997). The evolution of morphological diversity. Annual Review of Ecology and Systematics, 28, 129–152.
Fu, Y. (1997). Statistical test of neutrality of mutations against population growth hitchhiking and background selection. Genetics, 147, 915–925.
Garnier, S., Magniez-Jannin, F., Rasplus, J. Y., & Alibert, P. (2005). When morphometry meets genetics: Inferring the phylogeography of Carabus solieri using Fourier analyses of pronotum and male genitalia. Journal of Evolutionary Biology, 18, 269–280.
Gower, J. C. (1975). Generalized procrustes analysis. Psychometrika, 40, 33–51.
Hankison, S. J., & Ptacek, M. B. (2008). Geographical variation of genetic and phenotypic traits in the Mexican sailfin mollies, Poecilia velifera and P. petenensis. Molecular Ecology, 17, 2219–2233.
Hosken, D. J., & Stockley, P. (2004). Sexual selection and genital evolution. Trends in Ecology and Evolution, 19, 8793.
House, C. M., Lewis, Z., Hodgson, D. J., Wedell, N., Sharma, M. D., Hunt, J., & Hosken, D. J. (2013). Sexual and natural selection both influence male genital evolution. PLoS ONE, 8, e63807. https://doi.org/10.1371/journal.pone.0063807.
House, C. M., & Simmons, L. W. (2003). Genital morphology and fertilization success in the dung beetle Onthophagus taurus: An example of sexually selected male genitalia. Proceedings: Biological Sciences, 27, 447–455.
Hurtado, J., Iglesias, P. P., Lipko, P., & Hasson, E. (2013). Multiple paternity and sperm competition in the sibling species Drosophila buzzatii and Drosophila koepferae. Molecular Ecology, 22(19), 5016–5026.
Jackson, D. A. (1993). Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology, 74(8), 2204–2214.
Kamimura, Y. (2012). Correlated evolutionary changes in Drosophila female genitalia reduce the possible infection risk caused by male copulatory wounding. Behavioral Ecology and Sociobiology, 66, 1107–1114.
Leinonen, T., Cano, J. M., Mäkinen, H., & Merilä, J. (2006). Contrasting patterns of body shape and neutral genetic divergence in marine and lake populations of threespine sticklebacks. Journal of Evolutionary Biology, 19, 1803–1812.
Leinonen, T., Scott McCairns, R. J., O’Hara, R. B., & Merilä, J. (2013). QST – FST comparisons: Evolutionary and ecological insights from genomic heterogenity. Nature, 14, 179–190.
Lipko, P. (2013). Qué historias nos cuentan el ADN mitocondrial y los microsatélites sobre la estructura poblacional de Drosophila koepferae y su especie hermana Drosophila buzzatii en Argentina? Doctoral dissertation. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. http://digital.bl.fcen.uba.ar/Download/Tesis/Tesis_5434_Lipko.pdf.
Manfrin, M. H., De Brito, R. O. A., & Sene, F. M. (2001). Systematics and evolution of the Drosophila buzzatii (Diptera: Drosophilidae) cluster using mtDNA. Annals of the Entomological Society of America, 94, 334–346.
Manfrin, M. H., & Sene, F. M. (2006). Cactophilic Drosophila in South America: A model for evolutionary studies. Genetica, 126, 57–75.
Masly, J. P. (2012). 170 Years of “Lock-and-Key”: Genital morphology and reproductive isolation. International Journal of Evolutionary Biology, 2012, 247352. https://doi.org/10.1155/2012/247352.
Mayr, E. (1963). Animal species and evolution. Cambridge, MA: Harvard University Press.
McArdle, B. H., & Anderson, M. J. (2001). Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology, 82, 290–297.
McPeek, M. A., Shen, L., Torrey, J. Z., & Farid, H. (2008). The tempo and mode of three-dimensional morphological evolution in male reproductive structures. The American Naturalist, 171, 158–178.
Naveira, H., & Fontdevila, A. (1986). The evolutionary history of Drosophila buzzatii. XII. The genetic basis of sterility in hybrids between and its sibling from Argentina. Genetics, 114, 841–857.
Oksanen, J. F., Blanchet, G., Friendly, F., Kindt, R., Legendre, P., McGlinn, D., et al. (2017). Vegan: Community Ecology Package.
Oliveira, D. C. S. G., Almeida, F. C., O’ Grady, P. M., Armella, M. A., De Salle, R., & Etges, W. J. (2012). Monophyly, divergence times, and evolution of host plant use inferred from a revised phylogeny of the Drosophila repleta species group. Molecular Phylogenetics and Evolution, 64, 533–544.
Piccinali, R., Aguadé, M., & Hasson, E. (2004). Comparative molecular population genetics of the Xdh locus in the cactophilic sibling species Drosophila buzzatii and D. koepferae. Molecular Biology and Evolution, 21, 141–152.
Pujol, B., Wilson, A. J., Ross, R. I. C., & Pannell, J. R. (2008). Are QST—FST comparisons for natural populations meaningful? Molecular Ecology, 17, 4782–4785.
R Core Team. (2016). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/.
Raeymaekers, J. A. M., Van Houdt, J. K. J., Larmuseau, M. H. D., Geldof, S., & Volckaert, F. A. M. (2007). Divergent selection as revealed by PST and QTL-based FST in three-spined stickleback (Gasterosteus aculeatus) populations along a coastal-inland gradient. Molecular Ecology, 16, 891–905.
Richmond, M. P., Johnson, S., & Markov, T. A. (2012). Evolution of reproductive morphology among recently diverged taxa in the Drosophila mojavensis species cluster. Ecology and Evolution, 2, 397–408.
Rogers, A. R., & Harpending, H. (1992). Population growth makes waves in the distribution of pairwise genetic differences. Molecular Biology and Evolution, 9, 552–569.
Rousset, F. (1997). Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics, 145, 1219–1228.
Rowe, L., & Arnqvist, G. (2012). Sexual selection and the evolution of genital shape and complexity in water striders. Evolution, 66, 40–54.
Shapiro, A. M., & Porter, A. H. (1989). The lock-and-key hypothesis: Evolutionary and biosystematic interpretation of insect genitalia. Annual Review of Entomology, 34, 231–245.
Simmons, L. W. (2013). Sexual selection and genital evolution. Australian Journal of Entomology, 53, 1–17.
Simmons, L. W., House, C. M., Hunt, J., & Garcia-Gonzalez, F. (2009). Evolutionary response to sexual selection in male genital morphology. Current Biology, 19, 1442–1446.
Soto, E. M., Goenaga, J., Hurtado, J. P., & Hasson, E. (2012). Oviposition and performance in natural hosts in cactophilic Drosophila. Evolutionary Ecology, 26(4), 975–990.
Soto, I. M. (2012). Aedeagal divergence in sympatric populations of two sibling species of cactophilic Drosophila (Diptera: Drosophilidae): Evidence of character displacement? Neotropical Entomology, 41, 207–213.
Soto, I. M., Carreira, V. P., Corio, C., Padró, J., Soto, E. M., & Hasson, E. (2014). Differences in tolerance to host cactus alkaloids in Drosophila koepferae and D. buzzatii. PLoS ONE, 9(2), e88370. https://doi.org/10.1371/journal.pone.0088370.
Soto, I. M., Carreira, V. P., Fanara, J. J., & Hasson, E. (2007). Evolution of male genitalia: Environmental and genetic factors affecting genital morphology in sibling Drosophila species and their hybrids. BMC Evolutionary Biology, 7, 77.
Soto, I. M., Carreira, V. P., Soto, E. M., & Hasson, E. (2008b). Wing morphology and fluctuating asymmetry are dependent of the host plant in cactophilic Drosophila. Journal of Evolutionary Biology, 21(2), 598–609.
Soto, I. M., Carreira, V. P., Soto, E. M., Marquez, F., Lipko, P., & Hasson, E. (2013). Rapid divergent evolution of male genitalia among populations of Drosophila buzzatii. Evolutionary Biology, 40, 395–407.
Soto, I. M., Manfrin, M. H., & Hasson, E. (2008a). Host-dependent phenotypic plasticity of male genital morphology in cactophilic Drosophila. Journal of Zoological Systematics and Evolutionary Research, 46(4), 368–373.
Spitze, K. (1993). Population structure in Daphnia obtusa: Quantitative genetic and allozyme variation. Genetics, 135, 367–374.
Tabachnick, B. G., & Fidell, L. S. (2007). Using multivariate statistics (5th ed.). Boston, MA: Pearson International.
Tajima, F. (1989). Stastical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics, 123, 585–595.
Vilela, C. R. (1983). A revision of the Drosophila repleta species group (Diptera: Drosophilidae). Revista Brasileira de Entomologia, 27, 1–114.
Wojcieszek, J. M., & Simmons, L. W. (2012). Evidence for stabilizing selection and slow divergent evolution of males genitalia in a millipede (Antichiropus variabilis). Evolution, 66, 1138–1153.
Wojcieszek, J. M., & Simmons, L. W. (2013). Divergence in genital morphology may contribute to mechanical reproductive isolation in a millipede. Ecology and Evolution, 3, 334–343.
Wright, S. (1951). The genetical structure of populations. Annals of Eugenics, 15, 323–354.
Acknowledgements
This work was supported by the National Research Council of Argentina (CONICET – PIP 112201500100423CO) and by the National Agency for Scientific and Technological Promotion (PICT 1506–2013) and University of Buenos Aires grants (UBACyT GF2013-2016) with funds granted to IMS. MIS is recipient of postgraduate scholarships from Universidad de Buenos Aires. PMC and PPI are recipients of postgraduate scholarships from CONICET. EMS and IMS are members of Carrera del Investigador Científico (CONICET). Special thanks to Paula Lipko for the assistance with FST calculations and sequence data and to Maite Mascaró Miquela Jauregui and Fernando Nuno Simoes Dias Marques for their kind assistance during drafting of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary material
Below is the link to the electronic supplementary material.
11692_2018_9444_MOESM1_ESM.xls
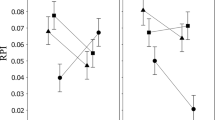
Plots of phenotypic (PST) differentiation compared to putative neutral genetic differentiation (FST) among populations for the male genital size (a) and first five shape variables (b–f). FST showed are those obtained from COI sequence (FSTCOI). Lines represent theoretical PST = FST as expected by neutral evolution (XLS 49 KB)
Rights and permissions
About this article
Cite this article
Stefanini, M.I., Milla Carmona, P., Iglesias, P.P. et al. Differential Rates of Male Genital Evolution in Sibling Species of Drosophila. Evol Biol 45, 211–222 (2018). https://doi.org/10.1007/s11692-018-9444-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-018-9444-0