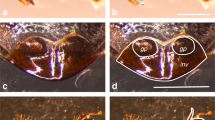
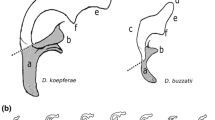
Abstract
Systematists and geneticists study biological diversity, but they use different approaches that rarely intersect. A very common pattern that is of interest for both researchers is the rapid evolution of genitalia, a trait of significant taxonomic utility in several sexually reproducing animal clades. The idea that both male and female genitalia are species-specific and play a role in reproductive isolation has long been controversial but has recently gained a renewed interest by speciation and developmental geneticists. Here, I highlight six unresolved questions in genitalia coevolution and I argue that systematists, with their well training in comparative morphology, usage of large and geographically diverse collections, and ability to apply molecular genetics techniques, can make important contributions. Such an extension of systematics into the speciation and developmental genetics realms is a promising opportunity to expand “integrative taxonomy” comparisons between DNA and morphology into more explanatory relationships between the two sources of taxonomic data.
Similar content being viewed by others
References
Arnqvist, G., & Rowe, L. (2002). Correlated evolution of male and female morphologies in water striders. Evolution, 56(5), 936–947.
Arnqvist, G., & Rowe, L. (2005). Sexual conflict. Princeton University Press.
Aspiras, A. C., Smith, F. W., & Angelini, D. R. (2011). Sex-specific gene interactions in the patterning of insect genitalia. Developmental Biology, 360(2), 369–380.
Baer, B., & Boomsma, J. J. (2006). Mating biology of the leaf-cutting ants Atta colombica and A. cephalotes. Journal of Morphology, 267(10), 1165–1171.
Bargielowski, I. E., Lounibos, L. P., & Carrasquilla, M. C. (2013). Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proceedings of the National Academy of Sciences of the United States of America, 110(8), 2888–2892.
Brennan, P. L. R., Prum, R. O., McCracken, K. G., Sorenson, M. D., Wilson, R. E., & Birkhead, T. R. (2007). Coevolution of male and female genital morphology in waterfowl. PLoS ONE, 2(5), e418.
Burla, H. (1954). Zur Kenntnis der Drosophiliden der Elfenbeinküste Französisch West-Afrika. Revue Suisse de Zoologie, 61(suppl), 1–218.
Cayetano, L., Maklakov, A. A., Brooks, R. C., & Bonduriansky, R. (2011). Evolution of male and female genitalia following release from sexual selection. Evolution, 65(8), 2171–2183.
Chatterjee, S. S., Uppendahl, L. D., Chowdhury, M. A., Ip, P.-L., & Siegal, M. L. (2011). The female-specific doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila. Development, 138(6), 1099–1109.
Cohn, M. J. (2011). Development of the external genitalia: conserved and divergent mechanisms of appendage patterning. Developmental Dynamics, 240(5), 1108–1115.
Coyne, J. A., & Orr, H. A. (2004). Speciation. Sinauer Associates, Incorporated Publishers.
Damen, W. G., & Tautz, D. (1999). Abdominal-B expression in a spider suggests a general role for Abdominal-B in specifying the genital structure. The Journal of Experimental Zoology, 285(1), 85–91.
Damen, W. G. M., Hausdorf, M., Seyfarth, E.-A., & Tautz, D. (1998). A conserved mode of head segmentation in arthropods revealed by the expression pattern of Hox genes in a spider. Proceedings of the National Academy of Sciences of the United States of America, 95(18), 10665–10670.
Dayrat, B. (2005). Towards integrative taxonomy. Biological Journal of the Linnean Society, 85(3), 407–415.
Drea, C. M., Place, N. J., Weldele, M. L., Coscia, E. M., Licht, P., & Glickman, S. E. (2002). Exposure to naturally circulating androgens during foetal life incurs direct reproductive costs in female spotted hyenas, but is prerequisite for male mating. Proceedings of the Royal Society B: Biological Sciences, 269(1504), 1981–1987.
Dufour, L. (1844). Anatomie générale des Diptères. Annales des Sciences Naturelles, 1, 224–264.
Dunkle, S. W. (1991). Head damage from mating attempts in dragonflies (Odonata: Anisoptera). Entomological News, 102(1), 37–41.
Duveau, F., & Félix, M.-A. (2012). Role of pleiotropy in the evolution of a cryptic developmental variation in Caenorhabditis elegans. PLoS Biology, 10(1), e1001230.
Eberhard, W. G. (1985). Sexual selection and Animal Genitalia. Harvard University Press.
Eberhard, W. G. (2004). Rapid divergent evolution of sexual morphology: comparative tests of antagonistic coevolution and traditional female choice. Evolution, 58(9), 1947–1970.
Eberhard, W. G. (2010a). Evolution of genitalia: theories, evidence, and new directions. Genetica, 138(1), 5–18.
Eberhard, W. G. (2010b). Rapid divergent evolution of genitalia: theory and data updated. In J. Leonard & A. Cordoba-Aguilar (Eds.), The evolution of primary sexual characters in animals (pp. 40–78). Oxford: Oxford University Press.
Eberhard, W. G., & Huber, B. A. (2010). Spider genitalia: precise maneuvers with a numb structure in a complex lock. In J. Leonard & A. Cordoba-Aguilar (Eds.), The evolution of primary sexual characters in animals (pp. 249–284). Oxford: Oxford University Press.
Eberhard, W., & Ramirez, N. (2004). Functional morphology of the male genitalia of four species of Drosophila: failure to confirm both lock and key and male–female conflict. Annals of the Entomological Society of America, 97(5), 1007–1017.
Evans, J. P., Gasparini, C., Holwell, G. I., Ramnarine, I. W., Pitcher, T. E., & Pilastro, A. (2011). Intraspecific evidence from guppies for correlated patterns of male and female genital trait diversification. Proceedings of the Royal Society B: Biological Sciences, 278(1718), 2611–2620.
Foronda, D., Estrada, B., de Navas, L., & Sánchez-Herrero, E. (2006). Requirement of Abdominal-A and Abdominal-B in the developing genitalia of Drosophila breaks the posterior downregulation rule. Development, 133(1), 117–127.
Garcia, L. R., LeBoeuf, B., & Koo, P. (2007). Diversity in mating behavior of hermaphroditic and male–female Caenorhabditis nematodes. Genetics, 175(4), 1761–1771.
Gavrilets, S. (2004). Fitness landscapes and the origin of species. Princeton, N.J.: Princeton University Press.
Gompert, Z., Nice, C. C., Fordyce, J. A., Forister, M. L., & Shapiro, A. M. (2006). Identifying units for conservation using molecular systematics: the cautionary tale of the Karner blue butterfly. Molecular Ecology, 15(7), 1759–1768.
Good, J. M., Demboski, J. R., Nagorsen, D. W., & Sullivan, J. (2003). Phylogeography and introgressive hybridization: chipmunks (genus Tamias) in the northern Rocky Mountains. Evolution, 57(8), 1900–1916.
Gorfinkiel, N., Sánchez, L., & Guerrero, I. (1999). Drosophila terminalia as an appendage-like structure. Mechanisms of Development, 86(1–2), 113–123.
Hasselquist, F. (1762). Reise nach Palästina in den Jahren 1749 bis 1752: Rostock.
Helsdingen, P. J. V. (1969). A reclassification of the species of Linyphia Latreille based on the functioning of the genitalia (Araneida, Linyphiidae). Brill Archive.
Hill, W. C. O. (1951). The external genitalia of the female chimpanzec; with observations on the mammary apparatus. Proceedings of the Zoological Society of London, 121(1), 133–145.
Hodgkin, J. (1983). Male phenotypes and mating efficiency in Caenorhabditis elegans. Genetics, 103(1), 43–64.
Hollander, J., Smadja, C. M., Butlin, R. K., & Reid, D. G. (2013). Genital divergence in sympatric sister snails. Journal of Evolutionary Biology, 26(1), 210–215.
Horton, D. R., & Lewis, T. M. (2011). Variation in male and female genitalia among ten species of North American Anthocoris (Hemiptera: Heteroptera: Anthocoridae). Annals of the Entomological Society of America, 104(6), 1260.
Israelson, G. (1972). Male copulatory organs of Macaronesian species of Aphanarthrum Wollaston. With designations of lectotypes and descriptions of new taxa (Col. Scolytidae). Insect Systematics and Evolution, 3(4), 17–257.
Jagadeeshan, S., & Singh, R. S. (2006). A time-sequence functional analysis of mating behaviour and genital coupling in Drosophila: role of cryptic female choice and male sex-drive in the evolution of male genitalia. Journal of Evolutionary Biology, 19(4), 1058–1070.
Johnson, C. (1972). Tandem linkage, sperm translocation, and copulation in the dragonfly, Hagenius brevistylus (Odonata: Gomphidae). American Midland Naturalist, 88(1), 131–149.
Jordal, B. H., Emerson, B. C., & Hewitt, G. M. (2006). Apparent “sympatric” speciation in ecologically similar herbivorous beetles facilitated by multiple colonizations of an island. Molecular Ecology, 15(10), 2935–2947.
Kameda, Y., Kawakita, A., & Kato, M. (2009). Reproductive character displacement in genital morphology in Satsuma land snails. The American Naturalist, 173(5), 689–697.
Kamimura, Y. (2007). Twin intromittent organs of Drosophila for traumatic insemination. Biology Letters, 3(4), 401–404.
Kamimura, Y., & Mitsumoto, H. (2011). Comparative copulation anatomy of the Drosophila melanogaster species complex (Diptera: Drosophilidae). Entomological Science, 14(4), 399–410.
Kamimura, Y., & Mitsumoto, H. (2012). Lock-and-key structural isolation between sibling Drosophila species. Entomological Science, 15(2), 197–201.
Kawakami, Y., & Tatsuta, H. (2010). Variation in the shape of genital appendages along a transect through sympatric and allopatric areas of two brachypterous grasshoppers, Parapodisma setouchiensis and Parapodisma subastris (Orthoptera: Podisminae). Annals of the Entomological Society of America, 103(3), 327–331.
Kawano, K. (2002). Character displacement in giant rhinoceros beetles. The American Naturalist, 159(3), 255–271.
Kawano, K. (2003). Character displacement in stag beetles (Coleoptera: Lucanidae). Annals of the Entomological Society of America, 96(4), 503–511.
Keisman, E. L., & Baker, B. S. (2001). The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development, 128(9), 1643–1656.
Khila, A., Abouheif, E., & Rowe, L. (2012). Function, developmental genetics, and fitness consequences of a sexually antagonistic trait. Science, 336(6081), 585–589.
Koene, J. M., & Schulenburg, H. (2005). Shooting darts: co-evolution and counter-adaptation in hermaphroditic snails. BMC Evolutionary Biology, 5(1), 25.
Liu, J., Mercer, J. M., Stam, L. F., Gibson, G. C., Zeng, Z. B., & Laurie, C. C. (1996). Genetic analysis of a morphological shape difference in the male genitalia of Drosophila simulans and D. mauritiana. Genetics, 142(4), 1129–1145.
Lucas, L. K., Fordyce, J. A., & Nice, C. C. (2008). Patterns of genitalic morphology around suture zones in North American Lycaeides (Lepidoptera: Lycaenidae): implications for taxonomy and historical biogeography. Annals of the Entomological Society of America, 101(1), 172–180.
Macagno, A. L. M., & Moczek, A. P. (2015). Appendage-patterning genes regulate male and female copulatory structures in horned beetles. Evolution & Development, 17(4), 248–253.
Macdonald, S. J., & Goldstein, D. B. (1999). A quantitative genetic analysis of male sexual traits distinguishing the sibling species Drosophila simulans and D. sechellia. Genetics, 153(4), 1683–1699.
Masly, J. P. (2012). 170 years of “lock-and-key”: genital morphology and reproductive isolation. International Journal of Evolutionary Biology, 2012, 1–10.
Matute, D. R., & Coyne, J. A. (2010). Intrinsic reproductive isolation between two sister species of Drosophila. Evolution, 64(4), 903–920.
Maupas, E. (1900). Modes et formes de reproduction des nématodes. Archives de Zoologie Expérimentale et Générale, 8, 463–624.
Mayr, E. (1963). Animal species and evolution. Cambridge: Belknap Press of Harvard University Press.
McLean, C. Y., Reno, P. L., Pollen, A. A., Bassan, A. I., Capellini, T. D., Guenther, C., et al. (2011). Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature, 471(7337), 216–219.
McNeil, C. L., Bain, C. L., & Macdonald, S. J. (2011). Multiple quantitative trait loci influence the shape of a male-specific genital structure in Drosophila melanogaster. G3: Genes, Genomes. Genetics, 1(5), 343–351.
McPeek, M. A., Shen, L., & Farid, H. (2009). The correlated evolution of three-dimensional reproductive structures between male and female damselflies. Evolution, 63(1), 73–83.
Meigen, J. W. (1830). Systematische Beschreibung der bekannten Europaischen zweiflugeligen insekten. Schulze.
Minelli, A. (2015). Biological systematics in the Evo-Devo era. European Journal of Taxonomy, 125, 1–23.
Nagata, N., Kubota, K., Yahiro, K., & Sota, T. (2007). Mechanical barriers to introgressive hybridization revealed by mitochondrial introgression patterns in Ohomopterus ground beetle assemblages. Molecular Ecology, 16(22), 4822–4836.
Nunes, M. D. S., Orozco-Ter Wengel, P., Kreissl, M., & Schlotterer, C. (2010). Multiple hybridization events between Drosophila simulans and Drosophila mauritiana are supported by mtDNA introgression. Molecular Ecology, 19(21), 4695–4707.
Paaby, A. B., & Rockman, M. V. (2013). The many faces of pleiotropy. Trends in Genetics, 29(2), 66–73.
Parsch, J., & Ellegren, H. (2013). The evolutionary causes and consequences of sex-biased gene expression. Nature Reviews Genetics, 14(2), 83–87.
Patterson, B. D., & Thaeler, C. S. (1982). The mammalian baculum: hypotheses on the nature of bacular variability. Journal of Mammalogy, 63(1), 1–15.
Peluffo, A. E., Nuez, I., Debat, V., Savisaar, R., Stern, D. L., & Orgogozo, V. (2015). A major locus controls a genital shape difference involved in reproductive isolation between Drosophila yakuba and Drosophila santomea. G3: Genes|Genomes|Genetics, g3.115.023481.
Peretti, A. V. (2003). Functional morphology of spermatophores and female genitalia in bothriurid scorpions: genital courtship, coercion and other possible mechanisms. Journal of Zoology, 261, 135–153.
Pitnick, S., Markow, T., & Spicer, G. S. (1999). Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution, 53(6), 1804–1822.
Pratt, H. L. (1979). Reproduction in the blue shark Prionace glauca. Fishery Bulletin, 77, 445–470.
Robertson, H. M., & Paterson, H. E. H. (1982). Mate recognition and mechanical isolation in Enallagma damselflies (Odonata: Coenagrionidae). Evolution, 36(2), 243–250.
Robson, G. C., & Richards, O. W. (1936). The Variation of Animals in Nature. London, New York [etc.] Longmans, Green and Co.
Rönn, J., Katvala, M., & Arnqvist, G. (2007). Coevolution between harmful male genitalia and female resistance in seed beetles. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 10921–10925.
Ryckman, R. E., & Ueshima, N. (1964). Biosystematics of the Hesperocimex complex (Hemiptera: Cimicidae) and avian hosts (Piciformes: Picidae; Passeriformes: Hirundinidae). Annals of the Entomological Society of America, 57, 624–638.
Sánchez, L., & Guerrero, I. (2001). The development of the Drosophila genital disc. BioEssays, 23(8), 698–707.
Sánchez, V., Hernández-Baños, B. E., & Cordero, C. (2011). The evolution of a female genital trait widely distributed in the Lepidoptera: comparative evidence for an effect of sexual coevolution. PLoS ONE, 6(8), e22642.
Sasabe, M., Takami, Y., & Sota, T. (2010). QTL for the species-specific male and female genital morphologies in Ohomopterus ground beetles. Molecular Ecology, 19(23), 5231–5239.
Sauer, J., & Hausdorf, B. (2009). Sexual selection is involved in speciation in a land snail radiation on crete. Evolution, 63(10), 2535–2546.
Schäfer, M. A., Routtu, J., Vieira, J., Hoikkala, A., Ritchie, M. G., & Schlötterer, C. (2011). Multiple quantitative trait loci influence intra-specific variation in genital morphology between phylogenetically distinct lines of Drosophila montana. Journal of Evolutionary Biology, 24(9), 1879–1886.
Schlick-Steiner, B. C., Steiner, F. M., Seifert, B., Stauffer, C., Christian, E., & Crozier, R. H. (2010). Integrative taxonomy: a multisource approach to exploring biodiversity. Annual Review of Entomology, 55(1), 421–438.
Shapiro, A. M., & Porter, A. H. (1989). The lock-and-key hypothesis: evolutionary and biosystematic interpretation of insect genitalia. Annual Review of Entomology, 34(1), 231–245.
Simmons, L. W., & Garcia-Gonzalez, F. (2011). Experimental coevolution of male and female genital morphology. Nature Communications, 2, 374.
Siva-Jothy, M. T. (2006). Trauma, disease and collateral damage: conflict in cimicids. Philosophical Transactions of the Royal Society, B: Biological Sciences, 361(1466), 269–275.
Skuse, F. A. A. (1894). The banded mosquito of Bengal. Indian Museum Notes, 3(5), 20.
Sota, T. (2002). Radiation and reticulation: extensive introgressive hybridization in the carabid beetles Ohomopterus inferred from mitochondrial gene genealogy. Population Ecology, 44(3), 145–156.
Sota, T., & Kubota, K. (1998). Genital lock-and-key as a selective agent against hybridization. Evolution, 52(5), 1507–1513.
Standfuss, M. R. (1896). Handbuch der paläarktischen Gross-Schmetterlinge für Forscher und Sammler. Jena : G. Fischer.
Sturtevant, A. H. (1919). A new species closely resembling Drosophila melanogaster. Psyche, 26(6), 153–155.
Takahara, B., & Takahashi, K. H. (2015). Genome-wide association study on male genital shape and size in Drosophila melanogaster. PLoS ONE, 10(7), e0132846.
Takami, Y., Nagata, N., Sasabe, M., & Sota, T. (2007). Asymmetry in reproductive isolation and its effect on directional mitochondrial introgression in the parapatric ground beetles Carabus yamato and C. albrechti. Population Ecology, 49(4), 337–346.
Tanabe, T., & Sota, T. (2008). Complex copulatory behavior and the proximate effect of genital and body size differences on mechanical reproductive isolation in the millipede genus Parafontaria. The American Naturalist, 171(5), 692–699.
Tanaka, K. M., Hopfen, C., Herbert, M. R., Schlötterer, C., Stern, D. L., Masly, J. P., et al. (2015). Genetic architecture and functional characterization of genes underlying the rapid diversification of male external genitalia between Drosophila simulans and Drosophila mauritiana. Genetics, 200(1), 357–369.
Tatarnic, N. J., & Cassis, G. (2010). Sexual coevolution in the traumatically inseminating plant bug genus Coridromius. Journal of Evolutionary Biology, 23(6), 1321–1326.
Toews, D. P. L., & Brelsford, A. (2012). The biogeography of mitochondrial and nuclear discordance in animals. Molecular Ecology, 21(16), 3907–3930.
True, J. R., Liu, J., Stam, L. F., Zeng, Z.-B., & Laurie, C. C. (1997). Quantitative genetic analysis of divergence in male secondary sexual traits between Drosophila simulans and Drosophila mauritiana. Evolution, 51(3), 816–832.
Tsacas, L., & Lachaise, D. (1974). Quatre nouvelles espèces de la Côte-d’Ivoire du genre Drosophila, groupe melanogaster, et discussion de l’origine du sous-groupe melanogaster (Diptera: Drosophilidae). Annales de l’Université d’Abidjan, 7, 193–211.
Uhl, G., Nessler, S. H., & Schneider, J. (2007). Copulatory mechanism in a sexually cannibalistic spider with genital mutilation (Araneae: Araneidae: Argiope bruennichi). Zoology, 110(5), 398–408.
Valdés, A. (2004). Morphology of the penial hooks and vaginal cuticular lining of some dorid nudibranchs (Mollusca, Opisthobranchia). American Malacological Bulletin, 18, 49–54.
Vanderplank, F. L. (1948). Experiments in crossbreeding tsetse-flies, Glossina species. Annals of Tropical Medicine and Parasitology, 42(2), 131–152.
Veuille, M. (1978). Biologie de la reproduction chez Jaera (Isopode Asellote) II. Evolution des organes reproducteurs femelles. Cahiers de Biologie Marine, 19, 385–395.
Ware, A. D., & Opell, B. D. (1989). A test of the mechanical isolation hypothesis in two similar spider species. Journal of Arachnology, 17(2), 149–162.
Wiens, J. J. (2001). Widespread loss of sexually selected traits: how the peacock lost its spots. Trends in Ecology & Evolution, 16(9), 517–523.
Will, K. W., Mishler, B. D., & Wheeler, Q. D. (2005). The perils of DNA barcoding and the need for integrative taxonomy. Systematic Biology, 54(5), 844–851.
Wollaston, T. V. (1860). On additions to the Madeiran Coleoptera. Annals and Magazine of Natural History, 3(5), 358–365.
Yassin, A., & David, J. R. (2016). Within-species reproductive costs affect the asymmetry of satyrization in Drosophila. Journal of Evolutionary Biology, 29(2), 455–460.
Yassin, A., & Orgogozo, V. (2013). Coevolution between male and female genitalia in the Drosophila melanogaster species subgroup. PLoS ONE, 8(2), e57158.
Zeng, Z.-B., Liu, J., Stam, L. F., Kao, C.-H., Mercer, J. M., & Laurie, C. C. (2000). Genetic architecture of a morphological shape difference between two Drosophila species. Genetics, 154(1), 299–310.
Acknowledgments
I am much grateful to an anonymous reviewer, Virginie Orgogozo, and Jean R. David for their constructive criticisms on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Yassin, A. Unresolved questions in genitalia coevolution: bridging taxonomy, speciation, and developmental genetics. Org Divers Evol 16, 681–688 (2016). https://doi.org/10.1007/s13127-016-0286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0286-2