Abstract
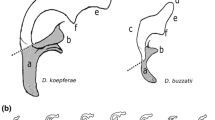
Increasing evidence from multiple animal systems suggests that genital evolution and diversification are driven by rapid and strong evolutionary forces. Particularly, the morphology of male genital structures is considered to be among the fastest evolving traits in animal groups with internal fertilization. In this study, we investigated patterns of male genital variation within and between natural populations of the cactophilic fly Drosophila buzzatii in its original geographic distribution range in the Neotropics. We detected significant morphological differences among populations and distinguished five differentiated groups. Moreover, among population differentiation in genital morphology was associated with the degree of geographic isolation among populations and clearly contrasted with the general homogeneity detected for the putatively neutral mitochondrial gene COI. Integrating our present data with previous molecular population genetic surveys, our results suggest that male genital morphology has rapidly diverged after the recent demographic expansion that D. buzzatii has undergone in the arid zones of South America. Because the “lock and key” hypothesis failed to explain the present pattern, we explored alternative explanations for the observed pattern of genital diversification including drift-facilitated sexual selection.





Similar content being viewed by others
References
Andrade, C. A. C., Hatadani, L. M., & Klaczko, L. B. (2005). Phenotypic plasticity of the aedeagus of Drosophila mediopunctata. Journal of Thermal Biology, 30, 518–523.
Andrade, C. A. C., Vieira, R. D., Ananina, G., & Klaczko, L. B. (2009). Evolution of the male genitalia: Morphological variation of the aedeagi in a natural population of Drosophila mediopunctata. Genetica, 135(1), 13–23.
Arnqvist, G. (1997). The evolution of animal genitalia: Distinguishing between hypotheses by single species studies. Biological Journal of the Linnean Society of London, 60, 365–379.
Brommer, J. E. (2011). Wither P ST? The approximation of Q ST by P ST in evolutionary and conservation biology. Journal of Evolutionary Biology, 24, 1160–1168.
Cabrera, A. L. (1976). Regiones Fitogeográficas Argentinas. In W. F. Kugler (Ed.), Enciclopedia Argentina de Agricultura y Jardinería (pp. 2–85). Buenos Aires: Acme.
Cavalcanti, M. J. (2008). Mantel for Windows. Test for association between two symmetric distance matrices with permutations iterations. Version 1.19 http://maurobio.infobio.net.
Coyne, J. A., & Orr, H. A. (2004). Speciation. Sunderland: Sinauer.
Crandall, K. A., Bininda-Emonds, O. R. P., Mace, G. M., & Wayne, R. K. (2000). Considering evolutionary processes in conservation biology. Trends in Ecology and Evolution, 15, 290–295.
Crespi, B. J. (2000). The evolution of maladaptation. Heredity, 84, 623–629.
De Brito, R. A., Manfrin, M. H., & Sene, F. M. (2002). Mitochondrial DNA phylogeography of Brazilian populations of Drosophila buzzatii. Genetics and Molecular Biology, 25(2), 161–171.
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M. & Robledo C. W. (2009). InfoStat ver. 2009. InfoStat Group, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar/.
Dufour, L. (1844). Anatomie générale des Diptères. Annuaire de Science Naturelle, 1, 244–264.
Endler, J. A. (1977). Geographic variation, speciation, and clines. Princeton: Princeton University Press.
Fanara, J. J., Folguera, G., Iriarte, P. F., Mensch, J., & Hasson, E. (2006). Genotype by environment interactions and development time in populations of cactophilic Drosophila. Journal of Evolutionary Biology, 19, 900–908.
Fernández Iriarte, P., & Hasson, E. (2000). The role of the use of different host plants in the maintenance of the inversion polymorphism in the cactophilic Drosophila buzzatii. Evolution, 54, 1295–1302.
Fernández Iriarte, P., Rodríguez, C., & Hasson, E. (2002). Inversion and allozyme polymorphism show contrasting patterns of microgeographic population structure in a natural population of Drosophila buzzatii. Journal of Evolutionary Biology, 15, 226–234.
Fontdevila, A., Ruiz, A., Ocaña, J., & Alonso, G. (1982). The evolutionary history of Drosophila buzzatii. II. How much has chromosomal polymorphism changed in colonization? Evolution, 36, 843–851.
Garnier, S., Magniez-Jannin, F., Rasplus, J. Y., & Alibert, P. (2005). When morphometry meets genetics: Inferring the phylogeography of Carabus solieri using Fourier analyses of pronotum and male genitalia. Journal of Evolutionary Biology, 18, 269–280.
Gómez, G. A., & Hasson, E. (2003). Transpecific polymorphisms in an inversion linked esterase locus in Drosophila buzzatii. Molecular Biology and Evolution, 20, 410–423.
Hasson, E., Fanara, J. J., Rodríguez, C., Vilardi, J. C., Reig, O. A., & Fontdevila, A. (1992). The evolutionary history of Drosophila buzzatii. XXIV: Second chromosome inversions have different average effect on thorax length. Heredity, 68, 557–563.
Hasson, E., Rodríguez, C., Fanara, J. J., Naveira, H., Reig, A. O., & Fontdevila, A. (1995). Macrogeographic patterns in the inversion polymorphisms of Drosophila buzzatii in New World populations. Journal of Evolutionary Biology, 8, 369–384.
Hasson, E., Soto, I. M., Carreira, V. P., Corio, C., Soto, E. M., & Betti, M. (2009). Host plants, fitness and developmental instability in a guild of cactophilic species of the genus Drosophila. In E. B. Santos (Ed.), Ecotoxicology research developments (pp. 89–109). Nueva York: Nova Science Publishers.
Hasson, E., Vilardi, J. C., Naveira, H., Fanara, J. J., Rodriguez, C., Reig, O. A., et al. (1991). The evolutionary history of Drosophila buzzatii. XVI. Fitness components analysis in a natural original population from Argentina. Journal of Evolutionary Biology, 4, 209–225.
Hedrick, P. W. (2005). Genetics of populations. Boston: Jones and Bartlett.
Hendry, A. P., Taylor, E. B., & McPhail, J. D. (2002). Adaptive divergence and the balance between selection and gene flow: Lake and stream stickleback in the Misty system. Evolution, 56, 1199–1216.
Hood, G. M. (2008). PopTools version 3.0.6. Available on the internet. URL http://www.cse.csiro.au/poptools.
Hosken, D. J., & Stockley, P. (2004). Sexual selection and genital evolution. Trends in Ecology and Evolution, 19, 8793.
House, C. M., & Simmons, L. W. (2003). Genital morphology and fertilization success in the dung beetle Onthophagus taurus: An example of sexually selected male genitalia. Proceedings of the Royal Society B, 270, 447–455.
Iwata, H., & Ukai, Y. (2002). SHAPE: A computer program package for quantitative evaluation of biological shapes based on Elliptic Fourier Descriptors. Journal of Heredity, 93, 384–385.
Jagadeeshan, S., & Singh, R. S. (2006). A time-sequence functional analysis of mating behaviour and genital coupling in Drosophila: Role of cryptic female choice and male sex-drive in the evolution of male genitalia. Journal of Evolutionary Biology, 19, 1058–1070.
King, R. B., & Lawson, R. (1995). Color-pattern variation in Lake Erie water snakes: The role of gene flow. Evolution, 49, 885–896.
Kopp, A., & True, J. R. (2002). Evolution of male sexual characters in the Oriental Drosophila melanogaster species group. Evolution and Development, 4, 278–291.
Kuhl, F. P., & Giardina, C. R. (1982). Elliptic Fourier features of a closed contour. Computer Graphics and Image Processing, 18, 236–258.
Laayouni, H., Hasson, E., Santos, M., & Fontdevila, A. (2003). The evolutionary history of Drosophila buzzatii. XXXV. Inversion polymorphism and nucleotide variability in different regions of the second chromosome. Molecular Biology and Evolution, 20, 931–944.
Leinonen, T., O’Hara, R., Cano, J. M., & Merilä, J. (2008). Comparative studies of quantitative trait and neutral marker divergence: A meta-analysis. Journal of Evolutionary Biology, 21, 1–17.
Lenormand, T. (2002). Gene flow and the limits to natural selection. Trends in Ecology and Evolution, 17, 183–189.
Lu, G., & Bernatchez, L. (1999). Correlated trophic specialization and genetic divergence in sympatric lake whitefish ecotypes (Coregonus clupeaformis): Support for the ecological speciation hypothesis. Evolution, 53, 1491–1505.
Manfrin, M. H., & Sene, F. M. (2006). Cactophilic Drosophila in South America: A model for evolutionary studies. Genetica, 126, 57–75.
Markow, T. A., & O’ Grady, P. M. (2006). Drosophila: A guide for species identification and use. London: Academic Press.
Masly, J. P. (2012). 170 years of “lock-and-key”: Genital morphology and reproductive isolation. International Journal of Evolutionary Biology,. doi:10.1155/2012/247352.
McPeek, M. A., Shen, L., Torrey, J. Z., & Farid, H. (2008). The tempo and mode of three-dimensional morphological evolution in male reproductive structures. American Naturalist, 171, 158–178.
Nei, M. (1972). Genetic distance between populations. American Naturalist, 106, 283–292.
Piccinali, R., Aguadé, M., & Hasson, E. (2004). Comparative molecular population genetics of the Xdh locus in the cactophilic sibling species Drosophila buzzatii and D. koepferae. Molecular Biology and Evolution, 21, 141–152.
Piccinali, R. V., Mascord, L. J., Barker, J. S., Oakeshott, J. G., & Hasson, E. (2007). Molecular population genetics of the alpha-esterase5 gene locus in original and colonized populations of Drosophila buzzatii and its sibling Drosophila koepferae. Journal of Molecular Evolution, 64, 158–170.
Polihronakis Richmond, M., Johnson, S., & Markow, T. A. (2012). Evolution of reproductive morphology among recently diverged taxa in the Drosophila mojavensis species cluster. Ecology and Evolution, 2(2), 397–408.
Pomiankowski, A., & Möller, A. P. (1995). A resolution of the lek paradox. Proceedings of the Royal Society B, 260, 21–29.
Pujol, B., Wilson, A. J., Ross, R. I. C., & Pannell, J. R. (2008). Are Q(ST)-F-ST comparisons for natural populations meaningful? Molecular Ecology, 17, 4782–4785.
Raeymaekers, J. A. M., Van Houdt, J. K. J., Larmuseau, M. H. D., Geldof, S., & Volckaert, F. A. M. (2007). Divergent selection as revealed by PST and QTL-based FST in three-spined stickle- back (Gasterosteus aculeatus) populations along a coastal-inland gradient. Molecular Ecology, 16, 891–905.
Riechert, S. E., Singer, F. D., & Jones, T. C. (2001). High gene flow levels lead to gamete wastage in a desert spider system. Genetica, 112, 297–319.
Rodríguez, C., Piccinali, R., Levy, E., & Hasson, E. (2000). Contrasting population genetic structures using allozymes and the inversion polymorphism in Drosophila buzzatii. Journal of Evolutionary Biology, 13, 976–984.
Rohlf, F. J., & Archie, J. W. (1984). A comparison of Fourier methods for the description of wing shape in mosquitoes (Diptera: Culicidae). Systematic Biology, 33, 302–317.
Ross, K. G., & Keller, L. (1995). Joint influence of gene flow and selection on a reproductively important genetic polymorphism in the fire ant Solenopsis invicta. American Naturalist, 146, 325–348.
Rossi, M. S., Barrio, E., Latorre, A., Quezada-Díaz, J. E., Hasson, E., Moya, A., et al. (1996). The evolutionary history of Drosophila buzzatii. XXX. Mitochondrial DNA polymorphism in original and colonizing populations. Molecular Biology and Evolution, 13, 314–323.
Saint-Laurent, R., Legault, M., & Bernatchez, L. (2003). Divergent selection maintains adaptive differentiation despite high gene flow between sympatric rainbow smelt ecotypes. Molecular Ecology, 12, 315–330.
Sandoval, C. P. (1994). The effects of relative geographic scales of gene flow and selection on morph frequencies in the walking stick Timema cristinae. Evolution, 48, 1866–1879.
Schluter, D. (1998). Ecological causes of speciation. In D. J. Howard & S. H. Berlocher (Eds.), Endless forms: Species and speciation (pp. 114–129). Oxford: Oxford University Press.
Schluter, D. (2000). The ecology of adaptive radiation. Oxford: Oxford University Press.
Slatkin, M. (1987). Gene flow and the geographic structure of natural populations. Science, 236, 787–792.
Smith, T. B., Wayne, R. K., Girman, D. J., & Bruford, M. W. (1997). A role for ecotones in generating rainforest biodiversity. Science, 276, 1855–1857.
Soto, I. M. (2012). Aedeagal divergence in sympatric populations of two sibling species of cactophilic Drosophila (Diptera, Drosophilidae): Evidence of character displacement? Neotropical Entomology, 41(3), 207–213.
Soto, I. M., Carreira, V. P., Fanara, J. J., & Hasson, E. (2007). Evolution of male genitalia: Environmental and genetic factors affecting genital morphology in sibling Drosophila species and their hybrids. BMC Evolutionary Biology, 7, 77.
Soto, I. M., Manfrin, M. H., & Hasson, E. (2008). Host-dependent phenotypic plasticity of male genital morphology in cactophilic Drosophila. Journal of Zoological Systematics and Evolutionary Research, 46, 368–373.
Soto, I. M., Soto, E. M., Carreira, V. P., Hurtado, J., Fanara, J. J., & Hasson, E. (2010). Geographic patterns of inversion polymorphism in the second chromosome of the cactophilic Drosophila buzzatii from northeastern Argentina. Journal of Insect Science, 10, 181.
Spitze, K. (1993). Population structure in Daphnia obtusa: Quantitative genetic and allozyme variation. Genetics, 135, 367–374.
StatSoft Inc. (2001). STATISTICA (data analysis software system), version 6.0. www.statsoft.com.
Storfer, A., Cross, J., Rush, R., & Caruso, J. (1999). Adaptive coloration and gene flow as a constraint to local adaptation in the streamside salamander, Ambystoma barbouri. Evolution, 53, 889–898.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10), 2731–2739.
Tazzyman, S. J., & Iwasa, Y. (2010). Sexual selection can increase the effect of random genetic drift—Quantitative genetic model of polymorphism in Oophaga pumilio, the strawberry poison-dart frog. Evolution, 64, 1558–5646.
Uyeda, J. C., Arnold, S. J., Hohenlohe, P. A., & Mead, L. S. (2009). Drift promotes speciation by sexual selection. Evolution, 63, 583–594.
Valdano, S. G. & Di Rienzo, J. (2007). Discovering meaningful groups in hierarchical cluster analysis. An extension to the multivariate case of a multiple comparison method based on cluster analysis. InterStat. http://interstat.statjournals.net/YEAR/2007/abstracts/0704002.php.
Vilela, C. R. (1983). A revision of the Drosophila repleta species group (Diptera, Drosophilidae). Revista Brasilera de Entomología, 27, 1–114.
Vilela, C. R., & Brito da Cunha, A. (2006). On marta breuer and some of her unpublished drawings of Drosophila spp. male terminalia (Diptera, Drosophilidae). Genetics and Molecular Biology, 587, 580–587.
Whitlock, M. C. (2008). Evolutionary inference from Qst. Molecular Ecology, 17, 1885–1896.
Wojcieszek, J. M., & Simmons, L. W. (2012). Evidence for stabilizing selection and slow divergent evolution of males genitalia in a millipede (Antichiropus variabilis). Evolution, 66(4), 1138–1153.
Acknowledgments
The authors wish to thank F. F. Franco and M. Polihronakis Richmond for helpful discussions in early stages of this investigation and in the drafting of the manuscript respectively. We are also grateful to two anonymous reviewers whose insightful advice and corrections helped to greatly improve the original manuscript. This work was supported by grants of Universidad de Buenos Aires, ANPCyT and CONICET. P. L., F. M. and E. M. S. are recipients of postgraduate and postdoctoral scholarships of CONICET respectively. I. M. S., V. P. C. and E. H. are members of Carrera del Investigador Científico (CONICET).
Conflict of interest
The authors also have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soto, I.M., Carreira, V.P., Soto, E.M. et al. Rapid Divergent Evolution of Male Genitalia Among Populations of Drosophila buzzatii . Evol Biol 40, 395–407 (2013). https://doi.org/10.1007/s11692-013-9223-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-013-9223-x