Abstract
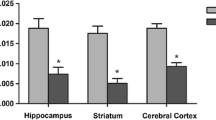
Maple syrup urine disease (MSUD) is characterized by a deficiency in the mitochondrial branched-chain α-keto acid dehydrogenase complex activity and, consequently, accumulation of the branched-chain amino acids and their respective branched-chain α-keto acids in fluids and the tissue. MSUD clinical symptoms include neurological alterations. KIC is considered one of the significant neurotoxic metabolites since its increased plasma concentrations are associated with neurological symptoms. We evaluated the effect of KIC intracerebroventricular (ICV) injection in hippocampal mitochondria function in rats. We also investigated the impact of KIC in cells’ metabolic activity (using MTT assay) and reactive species (RS) production in HT-22 cells. For this, thirty-day-old male rats were bilaterally ICV injected with KIC or aCSF. Thus, 1 hour after the administration, animals were euthanized, and the hippocampus was harvested for measured the activities of mitochondrial respiratory chain enzymes and RS production. Furthermore, HT-22 cells were incubated with KIC (1–10 mM) in 6, 12, and 24 h. Mitochondrial complexes activities were reduced, and the formation of RS was increased in the hippocampus of rats after KIC administration. Moreover, KIC reduced the cells’ metabolic ability to reduce MTT and increased RS production in hippocampal neurons. Impairment in hippocampal mitochondrial function seems to be involved in the neurotoxicity induced by KIC.


Similar content being viewed by others
References
Amaral AU, Leipnitz G, Fernandes CG, Seminotti B, Schuck PF, Wajner M (2010) Alpha-ketoisocaproic acid and leucine provoke mitochondrial bioenergetic dysfunction in rat brain. Brain Res 1324:75–84
Bridi R, Braun CA, Zorzi GK, Wannmacher CM, Wajner M, Lissi EG, Dutra-Filho CS (2005) Alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Metab Brain Dis 20:155–167
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Chuang DT, Shih VE (2001) Maple syrup urine disease (branched-chain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WL, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 1971–2005
de Castro Vasques V, de Boer MA, Diligenti F, Brinco F, Mallmann F, Mello CF, Wajner M (2004) Intrahippocampal administration of the alpha-keto acids accumulating in maple syrup urine disease provokes learning deficits in rats. Pharmacol Biochem Behav 77:183–190
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Funchal C, Latini A, Jacques-Silva MC, Dos Santos AQ, Buzin L, Gottfried C, Wajner M, Pessoa-Pureur R (2006) Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain alpha-keto acids accumulating in maple syrup urine disease. Neurochem Int 49:640–650
Halestrap AP, Brand MD, Denton RM (1974) Inhibition of mitochondrial pyruvate transport by phenylpyruvate and alpha-ketoisocaproate. Biochim Biophys Acta 367:102–108
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Howell RK, Lee M (1963) Influence of alpha-ketoacids on the respiration of brain in vitro. Proc Soc Exp Biol Med 113:660–663
Izquierdo I, Medina JH (1997) Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem 68:285–316
Jan W, Zimmerman RA, Wang ZJ, Berry GT, Kaplan PB, Kaye EM (2003) MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology 45:393–399
Jouvet P, Kozma M, Mehmet H. (2000). Primary human fibroblasts from a maple syrup urine disease patient undergo apoptosis following exposure to physiological concentrations of branched chain amino acids. Ann N Y Acad Sci. 926:116–21
Kann O, Kovács R (2007) Mitochondria and Neuronal Activity. Am J Physiol Cell Physiol 292:C641–C657
Lebel CP, Ali SF, McKee M, Bondy SC (1990) Organometal-induced increases in oxygen reactive species: The potential of 2’,7’- dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol 104:17–24
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mescka C, Moraes T, Rosa A, Mazzola P, Piccoli B, Jacques C, Dalazen G, Coelho J, Cortes M, Terra M, Regla Vargas C, Dutra-Filho CS (2011) In vivo neuroprotective effect of L-carnitine against oxidative stress in maple syrup urine disease. Metab Brain Dis 26:21–28
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front Cell Neurosci 13:363
Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. Academic Press, Cambridge
Rai Y, Pathak R, Kumari N, Sah DK, Pandey S, Kalra N, Soni R, Dwarakanath BS, Bhatt AN (2018) Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci Rep 8:1531
Ribeiro CA, Sgaravatti AM, Rosa RB, Schuck PF, Grando V, Schmidt AL, Ferreira GC, Perry ML, Dutra-Filho CS, Wajner M (2008) Inhibition of brain energy metabolism by the branched-chain amino acids accumulating in maple syrup urine disease. Neurochem Res 33:114–124
Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Scaini G, Tonon T, de Souza C, Schuk PF, Ferreira GC, Neto JS, Amorin T, Schwartz I, Streck EL (2017) Serum Markers of Neurodegeneration in Maple Syrup Urine Disease. Mol Neurobiol 54:5709–5719
Sgaravatti AM, Rosa RB, Schuck PF, Ribeiro CA, Wannmacher CM, Wyse AT, Dutra-Filho CS, Wajner M (2003) Inhibition of brain energy metabolism by the alpha-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta 1639:232–238
Sitta A, Ribas GS, Mescka CP, Barschak AG, Wajner M, Vargas CR (2014) Neurological damage in MSUD: the role of oxidative stress. Cell Mol Neurobiol 34:157–165
Snyderman SE, Norton PM, Roitman E, Holt LE (1964) Maple syrup urine disease with particular reference to diet therapy. Pediatrics 34:454–472
Stefanatos R, Sanz A (2018) The role of mitochondrial ROS in the aging brain. FEBS Lett 592:743–758
Strauss KA, Morton DH (2003) Branched-chain ketoacyl dehydrogenase deficiency: maple syrup urine disease. Curr Treat Options Neurol 5:329–341
Taschetto L, Scaini G, Zapelini HG, Ramos ÂC, Strapazzon G, Andrade VM, Réus GZ, Michels M, Dal-Pizzol F, Quevedo J, Schuck PF, Ferreira GC, Streck EL (2017) Acute and long-term effects of intracerebroventricular administration of α-ketoisocaproic acid on oxidative stress parameters and cognitive and noncognitive behaviors. Metab Brain Dis 32:1507–1518
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Voss JL, Bridge DJ, Cohen NJ, Walker JA (2017) A Closer Look at the Hippocampus and Memory. Trends Cogn Sci 21:577–588
Wisniewski MS, Carvalho-Silva M, Gomes LM, Zapelini HG, Schuck PF, Ferreira GC, Scaini G, Streck EL (2016) Intracerebroventricular administration of α-ketoisocaproic acid decreases brain-derived neurotrophic factor and nerve growth factor levels in brain of young rats. Metab Brain Dis 31:377–383
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological reviews 94:909–950
Acknowledgements
The authors would like to thank Prof. Marcelo Farina for donate HT-22 cells. This research was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação De Aperfeiçoamento De Pessoal De Nível Superior (CAPES), Universidade do Extremo Sul Catarinense (UNESC), and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have reviewed the contents of the manuscript, have a approved of its contents and validated the accuracy of the data and they have no financial or personal conflicts of interest related to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farias, H.R., Gabriel, J.R., Cecconi, M.L. et al. The metabolic effect of α-ketoisocaproic acid: in vivo and in vitro studies. Metab Brain Dis 36, 185–192 (2021). https://doi.org/10.1007/s11011-020-00626-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00626-y