Abstract
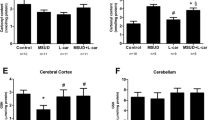
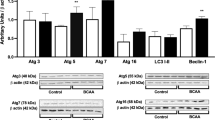
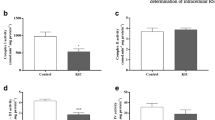
Maple syrup urine disease (MSUD) is an inherited neurometabolic disorder caused by deficiency of branched-chain α-keto acid dehydrogenase complex activity which leads to tissue accumulation of the branched-chain α-keto acids (BCKAs) α-ketoisocaproic acid (KIC), α-ketoisovaleric acid (KIV) and α-keto-β-methylvaleric acid (KMV) and their respective amino acids. Neuropathologic findings characteristic of the disease are cerebral edema and atrophy, whose pathophysiology is poorly known. In the present study, we investigated the in vitro effect of BCKAs on various parameters of oxidative stress, namely chemiluminescence (CL), thiobarbituric acid–reactive substances (TBA-RS), total radical-trapping antioxidant potential (TRAP), total antioxidant reactivity (TAR), and the activities of the antioxidant enzymes catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) in cerebral cortex of 30-day-old rats. The major effects observed were with KIC, which significantly increased CL and TBA-RS measurements, decreased TRAP and TAR values, and markedly inhibited GPx activity. KMV and KIV increased CL and decreased TRAP and TAR values. In contrast, these compounds did not affect CAT and SOD activities. Taken together, it was shown that: the BCKAs studied stimulated lipid peroxidation and reduced the brain antioxidant defences, suggesting an increased production of free radicals. In case the in vitro effects here detected also occur in vivo in MSUD, it can be presumed that oxidative stress might contribute, at least in part, to the brain damage found in the affected patients.
Similar content being viewed by others
References
Aebi, H. (1984). Catalase in vitro. Methods Enzymol. 105:121–126.
Annopkumar-Dukie, S., Lack, B., McPhail, T.N., Lambat, Z., Maharaj, D., and Daya, S. (2003). Indomethacin Reduces Lipid Peroxidation in Rat Brain Homogenate by Binding Fe2+. Metab Brain Dis. 18(1):1–9.
Araújo, P., Wassermann, G.F., Tallini, K., Furlanetto, V., Vargas, C.R., Wannmacher, C.M.D., Dutra-Filho, C.S., Wyse, A.T.S., and Wajner, M. (2001). Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem. Int. 38:529–537.
Aspée, A., and Lissi, E.A. (2000). Kinectics and mechanism of the chemiluminescence associated with the free radical-mediated oxidation amino acids. Luminescence 15:273–282.
Bridi, R., Araldi, J., Sgarbi, M.B., Testa, C.G., Durigon, K., Wajner, M., and Dutra-Filho, C.S. (2003). Induction of oxidative stress in rat brain by the metabolite accumulating in maple syrup urine disease. Int. J. Dev. Neurosci. 21:327–332.
Chuang, D.T., and Shih, V.E. (2001). Maple syrup urine disease (branched-chain ketoaciduria). In (C.R. Scriver, A.L. Beaudet, W.L. Sly, and D. Valle, eds.), The Metabolic and Molecular Bases of Inherited Disease, 8th edn., McGraw-Hill, New York, pp.1971–2005.
Cleeter, M.W., Cooper, J.M., and Shapira, A.H. (1992). Irreversible inhibition of mitocondrial complex I by 1-methyl-4-phenylpyrydinium: Evidence for free radical involvement. J. Neurochem. 58:786–789.
Danner, D.J., and Elsas, L.J. II (1989). Disorders of branched chain amino acid and keto acid metabolism. In (C.R. Scriver, A.L. Beaudet, W.L. Sly, and D. Valle, eds.), The Metabolic Basis of Inherited Disease, 6th edn., McGraw-Hill, New York, pp. 671–692.
Dodd, P.R., Wiliams, A.L., Gundlach, A.L., Harper, P.A.W., Healy, P.J., Dennis, J.A., and Johnston, G.A.R. (1992). Glutamate and γ-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J. Neurochem. 59:582–590.
Esterbauer, H., and Cheeseman, K.H. (1990). Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186:407–421.
Evelson, P., Travacio, M., Repetto, M., Escobar, J., Llesuy, S., and Lissi, E.A. (2001). Evaluation of total reactive antioxidant potential (trap) of tissue homogenates and their cytosols. Arch. Biochem. Biophys. 388:261–266.
Fontella, F.U., Gassen, E., Pulrolnik, V., Wannmacher, C.M.D., Klein, A.B., Wajner, M., and Dutra-Filho, C.S. (2002). Stimulation of lipid peroxidation in vitro in rat brain by metabolites accumulating in maple syrup urine disease. Metab Brain Dis. 17:47–54.
González-Flecha, B., Llesuy, S., and Boveris, A. (1991). Hydroperoxide-initiated chemiluminescence: An assay for oxidative stress in biopsies of heart, liver and muscle. Free Radic. Biol. Med. 10:93–100.
Halestrap, A.P., Brand, M.D., and Denton, R.M. (1974). Inhibition of mitochondrial pyruvate transport by phenylpyruvate and α-ketoisocaproate. Biochim. Biophys. Acta 367:102–108
Halliwell, B., and Gutteridge, J.M.C. (2001a). Detection of free radicals and others reactive species: Trapping and fingerprinting. In (B. Halliwell, and J.M.C. Gutteridge, eds.). Free Radicals in Biology and Medicine, Oxford University Press, Oxford, UK, pp. 351–425.
Halliwell, B., and Gutteridge, J.M.C. (2001b). The chemistry of free radicals and related ‘reactive species.’ In (B. Halliwell and J.M.C. Gutteridge, eds.). Free Radicals in Biology and Medicine, Oxford University Press, Oxford, UK, pp. 37–104.
Jouvet, P., Rustin, P., Taylor, D.L., Pocock, J.M., Felderhoff-Mueser, U., Mazarakis, N.D., Sarraf, C., Joashi, U., Koszma, M., Greewood, K., Edwards, A.D., and Mehmet, H. (2000). Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane despolarization or cytochrome c release: Implications for neurological impairment associated with maple syrup urine disease. Mol. Biol. Cell 11:1919–1932.
Kohen, R., Vellaichamy, E., Hrbac, J., Gati, I., and Tirosh, O. (2000). Quantification of the overall reactive oxygen species scavenging capacity of biological fluids and tissues. Free Radic. Biol. Med. 28(6):871–879.
Land, J.M., Mowbray, J., and Clark, J.B. (1976). Control of pyruvate and β-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J. Neurochem. 26:823–830.
Lissi, E., Pascual, C., and Del Castillo, M.D. (1992). Luminol luminescence induced by 2,2′-azo-bis-(2-amidinopropane) thermolysis. Free Radic. Res. 17:299–311.
Lissi, E., Salim-Hanna, M., Pascual, C., and Del Castillo, M.D. (1995). Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic. Biol. Med. 18:153–158.
Lissi, E.A., Cáceres, T., and Videla, L.A. (1988). Visible chemiluminescence from rat brain homogenates undergoing autoxidation. II. Kinetics of the luminescence decay. Free Radic. Biol. Med. 4:93–87.
Llesuy, S.F., Milei, J., Molina, H., Boveris, A., and Milei, S. (1985). Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4′-epiadriamycin in mice. Tumori 71:241–249.
Lowry, O.H., Rosebrough, N.J., Lewis-Farr, A., and Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Nyhan, W.L. (1984). Maple syrup urine disease: branched-chain ketoaciduria. In (W.L. Nyhan, ed.), Abnormalities in Amino Acid Metabolism in Clinical Medicine, Appleton-Century-Crofts, Norwalk, pp. 21–35.
Ogier de Baulny, H., and Saudubray, J.M. (2002). Branched-chain organic acidurias. Semin. Neonatol. 7(1):65–74.
Prensky, A.L., and Moser, H.W. (1967). Changes in the amino acid composition of proteolipids of white matter during maturation of the human nervous system. J. Neurochem. 14:117–121.
Rice, M.E., and Russo-Menna, I. (1998). Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience 82:1213–1223.
Saudubray, J.M., Ogier, H., Billette de Vilmeur, T., Bonnefont, J.P., Lyonnet, S., Herve, F., Munnich, A., Rabier, D., Coude, M., and Charpentier, C. (1991). Inborn errors of the metabolism of branched-chain amino acids. In (J. Schuab, F. van Hoof, H.L. Vis, eds.), Inborn Errors of Metabolism, Raven Press, New York, pp. 137–153.
Schadewaldt, P., and Wendel, U. (1997). Metabolism of branched-chain amino acids in maple syrup urine disease. Eur. J. Pediatr. 156:S62–S66.
Schapira, A.H.V. (1998). Human complex I defects in neurodegenerative diseases. Biochim. Biophys. Acta 1364:261–270.
Sgaravatti, A.M., Rosa, R.B., Schuck, P.F., Ribeiro, C.A.J., Wannacher, C.M.D., Wyse, A.T.S., Dutra-Filho, C.S., and Wajner, M. (2003). Inhibition of brain energy metabolism by the α-keto acids accumulating in maple syrup urine disease. Biochim. Biophys. Acta 1639:232–238.
Snyderman, S.E., Goldstein, F., Sansaricq, C., and Norton, P.M. (1984). The relationship between the branched chain amino acids and their α-ketoacids in maple syrup urine disease. Pediatr. Res. 18:851–853.
Snyderman, S.E., Norton, P.M., and Roitman, E. (1964). Maple syrup urine disease with particular reference to diet therapy. Pediatrics 34:454–472.
Tavares, R.G., Santos, C.E.S., Tasca, C., Wajner, M., Souza, D.O., and Dutra-Filho, C.S. (2000). Inhibition of glutamate uptake into synaptic vesicles of rat brain by the metabolites accumulating in maple syrup urine disease. J. Neurol. Sci. 181:44–49.
Tipton, K.F., and Singer, T.P. (1993). Advances in our understanding of the mechanisms of neurotoxicity of MPTP and related compounds. J. Neurochem. 61:1191–1206.
Treacy, E., Clow, C.L., Reade, T.R., Chitayat, D., Mamer, O.A., and Scriver, C.R. (1992). Maple syrup urine disease: Interrelationship between branched chain amino-, oxo-, and hydroxyacids; implications for treatment; association with CNS dysmelination. J. Inherit. Metab. Dis. 15:121–135.
Wendel, A. (1981). Glutathione peroxidase. Methods Enzymol. 77:325–332.
Yudkoff, M., Daikhin, Y., Lin, Z.P., Nissim, I., Stern, J., Pleasure, D., and Nissim, I. (1994). Interrelationships of leucine and glutamate metabolism in cultured astrocytes. J. Neurochem. 62:1192–1202.
Zielke, H.R., Huang, Y., Tildon, J.T., Zielke, C.L., and Baab, P.J. (1996). Elevation of amino acids in the interstitial space of the rat brain following infusion of large neutral amino and keto acids by microdialysis: Leucine infusion. Dev. Neurosci. 18:420–425.
Zielke, H.R., Zielke, C.L., Baab, P.J., and Collins, R.M. (2002). Large neutral amino acids auto exchange whwn infused by microdialysis into the rat brain: Implications for maple syrup urine disease and phenylketonuria. Neurochem. Int. 40:347–354.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bridi, R., Braun, C.A., Zorzi, G.K. et al. α-Keto Acids Accumulating in Maple Syrup Urine Disease Stimulate Lipid Peroxidation and Reduce Antioxidant Defences in Cerebral Cortex From Young Rats. Metab Brain Dis 20, 155–167 (2005). https://doi.org/10.1007/s11011-005-4152-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11011-005-4152-8