Abstract
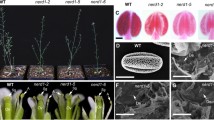
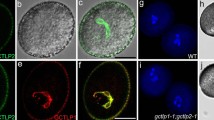
Arabidopsis Ruptured Pollen Grain-1 (RPG1/Sweet8) is a member of the MtN3/saliva protein family that functions as a sugar transporter. The rpg1 mutant shows defective exine pattern formation. In this study, transmission electron microscopy (TEM) observations showed that much less primexine was deposited in rpg1 tetrads. Furthermore, microspore membrane undulation was abnormal, and sporopollenin accumulation was also defective. This suggests that a reduced primexine deposition in rpg1 leads to abnormal membrane undulation that affects exine pattern formation. Chemical staining revealed thinning of the callose wall of rpg1, as well as significantly reduced expression of Callose synthase-5 (CalS5) in rpg1. The fertility of the rpg1 mutant could be partly restored at late reproductive stages, potentially complemented in part by RPG2, another member of the MtN3/saliva family, which is expressed in the anther during microsporogenesis. The double mutant, rpg1rpg2, was almost sterile and was not restored during late reproduction. These results suggest that RPG1 and RPG2 are involved in primexine deposition and therefore pollen wall pattern formation.





Similar content being viewed by others
References
Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A (1997) The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12:615–623
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181
Artero RD, Terol-Alcayde J, Paricio N, Ring J, Bargues M, Torres A, Perez-Alonso M (1998) Saliva, a new Drosophila gene expressed in the embryonic salivary glands with homologues in plants and vertebrates. Mech Dev 75:159–162
Chang HS, Zhang C, Chang YH, Zhu J, Xu XF, Shi ZH, Zhang XL, Xu L, Huang H, Zhang S, Yang ZN (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol 158:264–272
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ et al (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16:S84–S97
Fitzgerald MA, Knox RB (1995) Initiation of primexine in freeze substituted microspores of Brassica campestris. Sex Plant Reprod 8:99–104
Gabarayeva NI, Grigorjeva VV (2004) Exine development in Encephalartos altensteinii (Cycadaceae): ultrastructure, substructure and the modes of sporopollenin accumulation. Rev Palaeobot Palynol 132:175–193
Gabarayeva NI, Grigorjeva V, Rowley JR, Hemsley AR (2009) Sporoderm development in Trevesia burckii (Araliaceae). I. Tetrad period: further evidence for the participation of self-assembly processes. Rev Palaeobot Palynol 156:211–232
Gamas P, Niebel Fde C, Lescure N, Cullimore J (1996) Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol Plant Microbe Interact 9:233–242
Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN (2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147:852–863
Heslop-Harrison J (1968) Wall development within the microspore tetrad of Lilium longiflorum. Can J Bot 46:1185–1192
Heslop-Harrison J (1971) Wall pattern formation in angiosperm microsporogenesis. Symp Soc Exp Biol 25:277–300
Li XC, Zhu J, Yang J, Zhang GR, Xing WF, Zhang S, Yang ZN (2012) Glycerol-3-Phosphate acyltransferase 6 (GPAT6) is important for tapetum development in Arabidopsis and plays multiple roles in plant fertility. Mol plant 5:131–142
Nishikawa SI, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (β-1, 3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22
Paxson-Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127:1739–1749
Piffanelli P, Ross JHE, Murphy DJ (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80
Quilichini TD, Friedmann MC, Samuels AL, Douglas CJ (2010) ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis. Plant Physiol 154:678–690
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16:S46–S60
Southworth D, Jernstedt JA (1995) Pollen exine development precedes microtubule rearrangement in Vigna unguiculata (Fabaceae): a model for pollen wall patterning. Protoplasma 187:79–87
Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, Huang H, Xia HJ, Yang ZN (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52:528–538
Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou JT (2003) Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15:1872–1887
Acknowledgments
We thank the anonymous reviewers and Prof. Scott D. Russell for critical comments and language editing. This study was funded by grants from the National Science Foundation of China (30925007) and by the Leading Academic Discipline Project of Shanghai Municipal Education Commission (J50401).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Scott Russell.
M.-X. Sun, X.-Y. Huang and J. Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, MX., Huang, XY., Yang, J. et al. Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod 26, 83–91 (2013). https://doi.org/10.1007/s00497-012-0208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-012-0208-1