Abstract
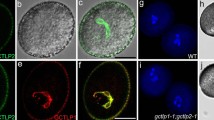
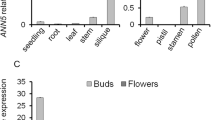
The primexine formation and plasma membrane undulation are the crucial steps of pollen wall formation in many angiosperms. However, the molecular mechanism underlining these processes is largely unknown. In Arabidopsis, NEW ENHANCER OF ROOT DWARFISM1 (NERD1), a transmembrane protein, was reported to play pleiotropic roles in plant development including male fertility control; while, how NERD1 disruption impacts male reproduction is yet unclear. Here, we revealed that the male sterility of nerd1 mutants is attributed to defects in early steps of pollen wall formation. We found that nerd1-2 is void of primexine formation and microspore plasma membrane undulation, defective in callose deposition. Consequently, sporopollenin precursors are unable to deposit and assemble on the microspore surface, but instead accumulated in the anther locule and tapetal cells, and ultimately leading to microspore abortion. NERD1 is localized in the Golgi and is expressed in both vegetative and reproductive organs, with the highest expression in reproductive tissues, including the tapetum, male meiocytes, tetrads and mature pollen grains. Our results suggest that NERD1 is required for the primexine deposition and microspore plasma membrane undulation, thus essential for sporopollenin assembly and pollen exine formation.








Similar content being viewed by others
Availability of data and material
The gene sequence of NERD1 is available in the tair website (http://www.arabidopsis.org/) under accession number AT3G51050. The supporting data of the article is included within the article and its additional files.
References
Aboulela M, Nakagawa T, Oshima A, Nishimura K, Tanaka Y (2018) The Arabidopsis COPII components, AtSEC23A and AtSEC23D, are essential for pollen wall development and exine patterning. J Exp Bot 69:1615–1633
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62:437–460
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181
Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K (2005) The HKM gene, which is identical to the MS1 gene of Arabidopsis thaliana, is essential for primexine formation and exine pattern formation. Sex Plant Reprod 18:1–7
Ariizumi T, Kawanabe T, Hatakeyama K, Sato S, Kato T, Tabata S, Toriyama K (2008) Ultrastructural characterization of exine development of the transient defective exine 1 mutant suggests the existence of a factor involved in constructing reticulate exine architecture from sporopollenin aggregates. Plant Cell Physiol 49:58–67
Chang HS, Zhang C, Chang YH, Zhu J, Xu XF, Shi ZH, Zhang XL, Xu L, Huang H, Zhang S, Yang ZN (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol 158:264–272
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B et al (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cole R, Peremyslov V, Van Why S, Moussaoui I, Ketter A, Cool R, Andres Moreno M, Vejlupkova Z, Dolja V, Fowler JE (2018) A broadly-conserved NERD genetically interacts with the exocyst to affect root growth and cell expansion. J Exp Bot 69:3625–3637
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DPS (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Feng ZY, Zhang BT, Ding WN, Liu XD, Yang DL, Wei PL, Cao FQ, Zhu SH, Zhang F, Mao Y, Zhu JK (2013) Efficient genome editing in plants using a CRISPR/Cas system. Cell Res 23:1229–1232
Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN (2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147:852–863
Heslop-Harrison J (1968) Wall development within the microspore tetrad of Lilium longiflorum. Can J Bot 46:1185–1192
Hong SK, Aoki T, Kitano H, Satoh H, Nagato Y (1995) Phenotypic diversity of 188 rice embryo mutants. Dev Genet 16:298–310
Hsieh K, Huang AH (2007) Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 19:582–596
Hu J, Wang Z, Zhang L, Sun MX (2014) The Arabidopsis Exine Formation Defect (EFD) gene is required for primexine patterning and is critical for pollen fertility. New Phytol 203:140–154
Ito T, Shinozaki K (2002) The MALE STERILITY1 gene of Arabidopsis, encoding a nuclear protein with a PHD-finger motif, is expressed in tapetal cells and is required for pollen maturation. Plant Cell Physiol 43:1285–1292
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Li WL, Liu Y, Douglas CJ (2017) Role of glycosyltransferases in pollen wall primexine formation and exine patterning. Plant Physiol 173:167–182
Ma L, Yang Z, Zhang S (2013) DEX1, a plasma membrane-localized protein, functions in microspore development by affecting CalS5 expression in Arabidopsis thaliana. Chin Sci Bull 58:2855–2861
Nishikawa SI, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (β-1, 3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22
Paxson-Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127:1739–1749
Piffanelli P, Ross JH, Murphy D (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80
Quilichini TD, Grienenberger E, Douglas CJ (2015) The biosynthesis, composition and assembly of the outer pollen wall: a tough case to crack. Phytochemistry 113:170–182
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Shi J, Cui M, Yang L, Kim YJ, Zhang D (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci 20:741–753
Sun MX, Huang XY, Yang J, Guan YF, Yang ZN (2013) Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod 26:83–91
Suzuki T, Narciso JO, Zeng W, van de Meene A, Yasutomi M, Takemura S, Lampugnani ER, Doblin MS, Bacic A, Ishiguro S (2017) KNS4/UPEX1: a type II arabinogalactan beta-(1,3)-galactosyltransferase required for pollen exine development. Plant Physiol 173:183–205
Tanaka Y, Nishimura K, Kawamukai M, Oshima A, Nakagawa T (2013) Redundant function of two Arabidopsis COPII components, AtSec24B and AtSec24C, is essential for male and female gametogenesis. Planta 238:561–575
Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 28:27–39
Xie HH, Chen L, Xu FQ, Guo WS, Wang S, Yang ZN, Zhang S (2017) ACOS5 is required for primexine formation and exine pattern formation during microsporogenesis in Arabidopsis. J Plant Biol 60:404–412
Xu T, Zhang C, Zhou Q, Yang ZN (2016) Pollen wall pattern in Arabidopsis. Sci Bull 61:832–837
Xu D, Shi J, Rautengarten C, Yang L, Qian X, Uzair M, Zhu L, Luo Q, An G, Waßmann F et al (2017) Defective Pollen Wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol 173:240–255
Yu J, Meng Z, Liang W, Kudla J, Tucker MR, Luo Z, Chen M, Xu D, Zhao G, Wang J et al (2016) A rice Ca2+ binding protein is required for tapetum function and pollen formation. Plant Physiol 172:1772–1786
Yuan J, Kessler SA (2019) A genome-wide association study reveals a novel regulator of ovule number and fertility in Arabidopsis thaliana. PLoS Genet 15:e1007934. https://doi.org/10.1371/journal.pgen.1007934
Zhao B, Shi H, Wang W, Liu X, Gao H, Wang X, Zhang Y, Yang M, Li R, Guo Y (2016) Secretory COPII protein SEC31B is required for pollen wall development. Plant Physiol 172:1625–1642
Acknowledgements
We are grateful to the Salk Genomic Analysis Laboratory for providing the T-DNA mutants seeds. The authors thank Wanwan Zhu, Ruifeng Fu and Lu Zhu for their help in TEM analysis. We thank Staffan Persson (University of Melbourne) for helpful comments and advice.
Funding
This research was supported by the National Key Research and Development Program of China (2016YFD0100903); the National Natural Science Foundation of China (U19A2031, 31670309, 31900611); China Innovative Research Team, Ministry of Education, and the Programme of Introducing Talents of Discipline to Universities (111 Project, B14016); JSPS KAKENHI Grant (JP19H05362).
Author information
Authors and Affiliations
Contributions
WQL, DBZ and DWX were involved in the design of the experiments and analyzed the data. DWX and PCM performed the experiments. IS helped for the immunolocalization experiment. DWX, DBZ, JXS and WQL wrote the manuscript. All authors have read, edited and approved publication of the present paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, D., Mondol, P.C., Ishiguro, S. et al. NERD1 is required for primexine formation and plasma membrane undulation during microsporogenesis in Arabidopsis thaliana. aBIOTECH 1, 205–218 (2020). https://doi.org/10.1007/s42994-020-00022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42994-020-00022-1