Summary
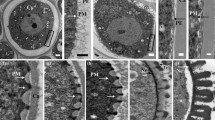
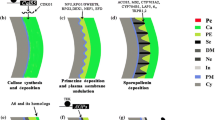
In order to identify factors necessary for the establishment of the reticulate pollen wall pattern, we have characterized a T-DNA tagged mutant ofArabidopsis thaliana that is defective in pattern formation. This study reports the results of an ultrastructural comparison of pollen wall formation in the mutant to wall development in wild-type plants. Pollen wall development in the mutant parallels that of wild-type until the early tetrad stage. At this point in wild-type plants, the microspore plasma membrane assumes a regular pattern of ridges and valleys. Initial sporopollenin deposition occurs on the ridges marking the beginning of probacula formation. In contrast, the plasma membrane in the mutant appears irregular with flattened protuberances and rare invaginations. As a result, the wild-type regular pattern of ridges and valleys is not formed. Sporopollenin is randomly deposited on the plasma membrane and aggregates on the locule wall; it is not anchored to the membrane. Our finding that the mutation blocks the normal invagination of the plasma membrane and disrupts the proper deposition of sporopollenin during wall formation suggests that the mutation could be in a gene responsible for pattern formation. These results also provide direct evidence that the plasma membrane plays a critical role in the establishment of the pollen wall pattern.
Similar content being viewed by others
References
Cutter EG (1971) Plant anatomy: experiment and interpretation. Addison-Wesley, Reading, MA
Dahl AO (1986) Observation on pollen development inArabidopsis under gravitationally controlled environments. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 49–60
Dickinson HG (1970) Ultrastructural aspects of primexine formation in the microspore tetrad ofLilium longiflorum. Cytobiologie 4: 437–449
—, Sheldon JM (1986) The generation of patterning at the plasma membrane of the young microspore ofLilium. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, p 1–17
Erdtman G (1952) Pollen morphology and plant taxonomy: an introduction to palynology. Almqvist and Wiksell, Stockholm
— (1969) Handbook of palynology: an introduction to the study of pollen grains and spores. Hafner, New York
Feldmann KA (1991) T-DNA insertion mutagenesis inArabidopsis: mutational spectrum. Plant J 1: 71–82
Fitzgerald MA, Knox RB (1995) Initiation of primexine in freeze-substituted microspores ofBrassica campestris. Sex Plant Reprod 8: 99–104
—, Barnes SH, Blackmore S, Calder DM, Knox RB (1994) Exine formation in the pollinium ofDendrobium. Protoplasma 179: 121–130
Godwin H (1968) The origin of the exine. New Phytol 67: 667–676
Heslop-Harrison J (1963) An ultrastructural study of pollen wall ontogeny inSilene pendula. Grana Palynol 4: 7–24
— (1971a) The pollen wall: structure and development. In: Heslop-Harrison J (ed) Pollen: development and physiology. Butterworths, London, pp 75–98
(1971b) Wall pattern formation in angiosperm microsporogenesis. Symp Soc Exp Biol 25: 277–300
Hoefert LL (1968) Polychromatic stains for the thin sections ofBeta embedded in epoxy resin. Stain Tectmol 43: 145–151
Ingber I (1993) Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. I Cell Sci 104: 613–627
Kuang A, Musgrave ME (1996) Dynamics of vegetative cytoplasm during generative cell formation and pollen maturation inArabidopsis thaliana. Protoplasma 194: 81–90
Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis inArabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae). Protoplasma 185: 7–21
Peirson BN, Owen HA, Feldmann KA, Makaroff CA (1996) Characterization of three male-sterile mutants ofArabidopsis thaliana exhibiting alterations in meiosis. Sex Plant Reprod 9: 1–16
Pérez-Muñoz CA, Jernstedt JA, Webster BD (1993) Pollen wall development inVigna vexillata II. Ultrastructural studies. Am J Bot 80: 1193–1202
—, Webster BD, Jernstedt JA (1995) Spatial congruence between exine pattern, microtubules and endomembranes inVigna pollen. Sex Plant Reprod 8: 147–151
Scott RJ (1994) Pollen exine — the sporopollenin enigma and the physics of pattern. In: Scott RJ, Stead MA (eds) Molecular and cellular aspects of plant reproduction. Cambridge University Press, Cambridge, pp 49–81
Sheldon JM, Dickinson HG (1983) Determination of patterning in the pollen wall ofLilium henryi. J Cell Sci 63: 191–208
— — (1986) Pollen wall formation inLilium: the effect of chaotropic agents, and the organisation of the microtubular cytoskeleton during pattern development. Planta 168: 11–23
Skvarla JJ, Larson DA (1966) Fine structural studies ofZea mays pollen I. Cell membranes and exine ontogeny. Am J Bot 53: 1112–1125
—, Rowley JR (1987) Ontogeny of pollen inPoinciana (Leguminoseae). I. Development of exine template. Rev Palaeobot Palynol 50: 293–311
Smith MM, McCully ME (1978) A critical evaluation of the specificity of aniline blue induced fluorescence. Protoplasma 95: 229–254
Southworth D, Jernstedt JA (1995) Pollen exine development precedes microtubule rearrangement inVigna unguiculata (Fabaceae): a model for pollen wall patterning. Protoplasma 187: 79–87
Stanley RG, Linskens HF (1974) Pollen: biology, biochemistry, management. Springer, New York Berlin Heidelberg
Sutherland J, McCully ME (1976) A note on the structural changes in the walls of pericycle cells initiating lateral root meristems inZea mays. Can J Bot 54: 2083–2087
Takahashi M (1989) Pattern determination of the exine inCaesalpinia japonica (Leguminosae: Caesalpinioideae). Am J Bot 76: 1615–1626
Takahashi M, Skvarla JJ (1991) Exine pattern formation by plasma membrane inBougainvillea spectabilis Willd. (Nyctaginaceae). Am J Bot 78: 1063–1069
Waterkeyn L, Bienfait A (1970) On a possible function of the callosic special wall inIpomoea purpurea (L) Roth. Grana 10: 13–20
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4: 759–771
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paxson-Sowders, D.M., Owen, H.A. & Makaroff, C.A. A comparative ultrastructural analysis of exine pattern development in wild-typeArabidopsis and a mutant defective in pattern formation. Protoplasma 198, 53–65 (1997). https://doi.org/10.1007/BF01282131
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01282131