Abstract
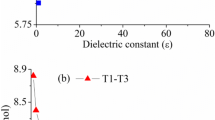
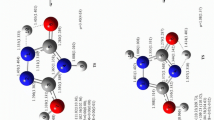
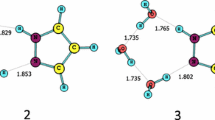
Electronic structures of allophanic acid were studied using MP2/6-311++G(d,p) level of theory. Five most stable tautomers were selected and stability of them was studied in detail. Obtained data showed that tautomer 1 having hydrogen atom in the central nitrogen N3 and also in a trans conformation of carbonyl and amino functional groups becomes the most stable one. Then, interconversion of these tautomers to each other was investigated step by step through internal rotation and proton transfer routes. Results indicated that movement of protons determines rate-determining step of all paths A–E. Effects of different solvents were carefully surveyed for each tautomer, and among investigated solvents, water made slight stabilization. Activation barrier for proton exchange assisted by one water molecule and one formic acid molecule was also studied, separately. Both molecules had a great influence on lowering barrier especially for proton transfer routes. Comparative MP2 study showed that the barrier will be lowered much more when acid-assisted proton exchange takes place.




















Similar content being viewed by others
References
Umemoto S, Takamatsu H (1963) Syntheses of 4-(phenylacetyl)allophanic acid ester derivatives. J Pharm Soc Jpn 83:917–920
Fan C, Li Z, Yin H, Xiang S (2013) Structure and function of allophanate hydrolase. J Biol Chem 288(29):21422–21432. doi:10.1074/jbc.M113.453837
Whitney PA, Cooper TG (1972) Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate: urea amido-lyase in Saccharomyces cerevisiae. J Biol Chem 247(5):1349–1353
Leftley JW, Syrett PJ (1973) Urease and ATP: urea amidolyase activity in unicellular algae. J Gen Microbiol 77(1):109–115
Maitz GS, Haas EM, Castric PA (1982) Purification and properties of the allophanate hydrolase from Chlamydomonas reinhardtii. Biochim Biophys (BBA) Gen Subj 714(3):486–491. doi:10.1016/0304-4165(82)90158-1
Schure EG, van Riel NAW, Verrips CT (2000) The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol Rev 24(1):67–83. doi:10.1016/S0168-6445(99)00030-3
Fukushima M, Miura A, Sasaki M, Izumo K (2009) Effect of an allophanic soil on humification reactions between catechol and glycine: spectroscopic investigations of reaction products. J Mol Struct 917(2–3):142–147. doi:10.1016/j.molstruc.2008.07.006
Ishiguro M, Nakajima T (2000) Hydraulic conductivity of an allophanic Andisol leached with dilute acid solutions. Soil Sci Soc Am J 64(3):813–818
Hashimoto Y, Kang J, Matsuyama N, Saigusa M (2012) Path analysis of phosphorus retention capacity in allophanic and non-allophanic andisols. Soil Sci Soc Am J 76(2):441–448. doi:10.2136/sssaj.2011.0196
Sluka J, Zikan V, Sova M, Danek J (1989) Derivatives of thioallophanic acid with an anthelmintic effect. Ceska Slov Farm 38(9):414–417
Yoo HS, Cooper TG (1985) Identification of sequences required for positive control of an allophanate-inducible gene in yeast. Fed Proc 44(5):1438
Nguyen TH, Hoang VH, Hoang-Do NT, Le VH (2012) Possibility of tracking imino–amino tautomerism of cytosine by ultra-short laser pulses using high-order harmonic generation. Comput Theor Chem 988:92–97. doi:10.1016/j.comptc.2012.02.037
Qg Li, Xue Y, Gs Yan (2008) Water-assisted enol-to-keto tautomerism of a simple peptide model: a computational investigation. J Mol Struct THEOCHEM 868(1–3):55–64. doi:10.1016/j.theochem.2008.08.004
Hoang VH, Le CT, Nguyen NT, Le VH (2014) Possibility of distinguishing DNA bases and of tracking the keto-enol tautomerism by using high-order harmonic generation. Comput Theor Chem 1043:31–37. doi:10.1016/j.comptc.2014.05.011
Jayaram PN, Roy G, Mugesh G (2008) Effect of thione—thiol tautomerism on the inhibition of lactoperoxidase by anti-thyroid drugs and their analogues. J Chem Sci 120(1):143–154. doi:10.1007/s12039-008-0017-0
Trujillo C, Mó O, Yáñez M (2007) A theoretical study of hydration effects on the prototropic tautomerism of selenouracils. Org Biomol Chem 5(19):3092–3099. doi:10.1039/b708045j
Qiu ZM, Wang GL, Wang HL, Xi HP, Hou D (2014) MP2 study on the hydrogen-bonding interaction between 5-fluorouracil and DNA bases: A, C, G, T. Struct Chem 25(5):1465–1474. doi:10.1007/s11224-014-0427-1
Qiu ZM, Wang HL, Liu YZ, Hou DN (2014) MP2 study on the hydrogen-bonding interaction between O 4-methylthymine and DNA bases: A, C, G, and T. Struct Chem 25(3):767–774. doi:10.1007/s11224-013-0335-9
Qiu ZM, Xi HP, Zhang SS, Li XD, Hou DN (2015) MP2 study on the hydrogen-bonding interaction between 5-fluorocytosine and DNA bases: A, C, G, T. Struct Chem. doi:10.1007/s11224-015-0624-6
Kaur D, Sharma R (2014) Insight into the acidic behavior of oxazolidin-2-one, its thione and selone analogs through computational techniques. Struct Chem 25(4):1111–1132. doi:10.1007/s11224-013-0382-2
Khanna S, Kaur D, Kaur R (2014) The saturated five-membered heterocyclic molecules as organic hydride donors: a computational study. J Phys Org Chem 27(9):747–755. doi:10.1002/poc.3334
Kaur D, Kaur R, Khanna S (2014) Impacts of medium, substituents, and specific interactions with water on hydration of carbonyl compounds. Struct Chem 25(2):437–450. doi:10.1007/s11224-013-0308-z
Kaur D, Khanna S (2012) Theoretical study on the hydrogen bonding of five-membered heteroaromatics with water. Struct Chem 23(3):755–764. doi:10.1007/s11224-011-9917-6
Liang X, Zheng W, Wong NB, Shu Y, Tian A (2005) Solvent-assisted catalysis mechanism on Keto–enol tautomerism of cyameluric acid. J Mol Struct THEOCHEM 732(1–3):127–137. doi:10.1016/j.theochem.2005.05.009
Valadbeigi Y, Farrokhpour H (2013) Theoretical study on keto–enol tautomerism and isomerization in pyruvic acid. Int J Quantum Chem 113(21):2372–2378. doi:10.1002/qua.24467
Wang G, Wang J, Nie H, Tan SP, Shi WQ, Zhao DM, Cheng MS (2013) Experimental and theoretical investigations on the tautomerism of 1-phenyl-2-thiobarbituric acid and its methylation reaction. J Mol Struct 1036:372–379. doi:10.1016/j.molstruc.2012.12.017
Zhang Z, Zhang J, Zheng Q, Kong C, Li Z, Zhang H, Ma J (2015) Theoretical investigation on binding process of allophanate to allophanate hydrolase. Chem Res Chin Univ 31(6):1023–1028. doi:10.1007/s40242-015-5108-0
Chahkandi B, Tayyari SF, Bakhshaei M, Chahkandi M (2013) Investigation of simple and water assisted tautomerism in a derivative of 1,3,4-oxadiazole: a DFT study. J Mol Graph Model 44:120–128. doi:10.1016/j.jmgm.2013.04.002
Trujillo C, Sánchez-Sanz G, Alkorta I, Elguero J (2015) Computational study of proton transfer in tautomers of 3- and 5-hydroxypyrazole assisted by water. ChemPhysChem 16(10):2140–2150. doi:10.1002/cphc.201500317
Dedíková P, Neogrády P, Urban M (2011) Electron affinities of small uracil-water complexes: a comparison of benchmark CCSD(T) calculations with DFT. J Phys Chem A 115(11):2350–2358. doi:10.1021/jp111104j
Hajipour AR, Chermahini AN, Karimzadeh M, Rezapour M (2015) Tautomerism and mechanism of intramolecular proton transfer under the gas phase and micro-hydrated solvent conditions: biuret as a case study. Struct Chem 26(1):159–169. doi:10.1007/s11224-014-0408-4
Hajipour AR, Karimzadeh M, Jalilvand S, Farrokhpour H, Chermahini AN (2014) A complete scheme of tautomerism on diacetyl monoxime in the gas and solution phases. A comparative DFT study between B3LYP and M06-2X functionals. Comput Theor Chem 1045:10–21. doi:10.1016/j.comptc.2014.05.019
Head-Gordon M, Pople JA, Frisch MJ (1988) MP2 energy evaluation by direct methods. Chem Phys Lett 153(6):503–506. doi:10.1016/0009-2614(88)85250-3
Head-Gordon M, Head-Gordon T (1994) Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer. Chem Phys Lett 220(1–2):122–128. doi:10.1016/0009-2614(94)00116-2
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to Isotropic and anisotropic dielectrics. J Chem Phys 107(8):3032–3041
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19(4):404–417
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94(7):2027–2094
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) Using redundant internal coordinates to optimize equilibrium geometries and transition states. J Comput Chem 17(1):49–56
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision B.01. Wallingford CT.
Chermahini AN, Teimouri A (2014) Theoretical studies on proton transfer reaction of 3(5)-substituted pyrazoles. J Chem Sci 126(1):273–281
Chermhini AN, Farrokhpour H, Teimouri A, Pourmoghaddas F (2013) Theoretical studies on tautomerism of imidazole-2-selenone. Struct Chem 24(4):1215–1227. doi:10.1007/s11224-012-0153-5
Chermahini AN, Teimouri A, Salimi Beni A, Dordahan F (2013) Theoretical studies on the effect of substituent in the proton transfer reaction of 4-substituted pyrazoles. Comput Theor Chem 1008:67–73. doi:10.1016/j.comptc.2012.12.021
Beni AS, Chermahini AN, Sharghi H, Monfared SM (2011) MP2, DFT and ab initio calculations on thioxanthone. Spectrochim Acta A 82(1):49–55. doi:10.1016/j.saa.2011.06.059
Beni AS, Dalirnasab Z, Teimouri A, Chermahini AN (2011) Studies on tautomerism in the triazoline dione. Can J Chem 89(11):1387–1395. doi:10.1139/v11-114
Chermahini AN, Teimouri A, Beni AS (2011) Theoretical studies on tautomerism of tetrazole 5-thion. Struct Chem 22(1):175–181. doi:10.1007/s11224-010-9704-9
Chermahini AN, Dabbagh HA, Teimouri A (2008) Theoretical studies on tautomerism of dihydropyrimidine tautomers. J Mol Struct THEOCHEM 857(1–3):105–110. doi:10.1016/j.theochem.2008.02.012
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hajipour, A.R., Karimzadeh, M., Ghorbani, S. et al. A comparative MP2 study between water- and acid-assisted proton transfer: allophanic acid as a case of study. Struct Chem 27, 1345–1362 (2016). https://doi.org/10.1007/s11224-016-0753-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0753-6