Abstract
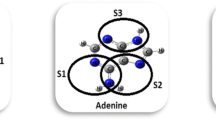
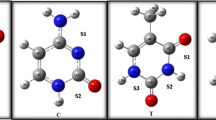
The 5-fluorouracil is a pyrimidine analog effective in the treatment of cancer. In this work, we present the hydrogen-bonding base pairs involving 5-FU bound to the four bases in DNA: adenine, cytosine, guanine, and thymine. Full geometry optimizations have been performed for the studied complexes by MP2 method. The interaction energies were corrected for the basis-set superposition error, using the full Boys-Bernardi counterpoise correction scheme. Hydrogen-bonding patterns of these base pairs were characterized using NBO analysis and AIM analysis. According to the calculated binding energies and structural parameters, the stability of the base pairs decrease in the following order: 5-FU:A > 5-FU:G > 5-FU:T > 5-FU:C.




Similar content being viewed by others
References
Watson JD, Crick FHC (1953) Nature 171:737–738. doi:10.1038/171738a0
Parthasarathi R, Subramanian V (2006) Chem Phys Lett 418:530–534. doi:10.1016/j.cplett.2005.10.153
Dkhissi A, Blossey R (2007) Chem Phys Lett 439:35–39. doi:10.1016/j.cplett.03.065
Thiviyanathan V, Somasunderam A, Volk DE, Hazra TK, Mitra S, Gorenstein DG (2008) Biochem Biophys Res Commun 366:752–757. doi:10.1016/j.bbrc.2007.12.010
Padermshoke A, Katsumoto Y, Masaki R, Aida M (2008) Chem Phys Lett 457:232–236. doi:10.1016/j.cplett.2008.04.029
Qiu ZM, Wang HJ, Xia YM (2010) Struct Chem 21:931–937. doi:10.1007/s11224-010-9629-3
Villani G (2006) Chem Phys 324:438–446. doi:10.1016/j.chemphys.2005.11.006
Grunenberg J (2004) J Am Chem Soc 126:16310–16311. doi:10.1021/ja046282a
Bhattacharyya D, Koripella SC, Mitra A, Rajendran VB, Sinha B (2007) J Biosci 32:809–825. doi:10.1007/s12038-007-0082-4
Qiu ZM, Xia YM, Wang HJ, Diao KS (2010) Struct Chem 21:99–105. doi:10.1007/s11224-009-9528-7
Šponer J, Leszczynski J, Hobza P (2001) Biopolymers 61(1):3–31. doi:10.1002/1097-0282
Brovarets’ OO, Hovorun DM (2014) J Biomol Struct Dyn 32(1):127–154. doi:10.1080/07391102.2012.755795
Brovarets’ OO, Yurenko YP, Hovorun DM (2013) J Biomol Struct Dyn 32:993–1022. doi:10.1080/07391102.2013.799439
Brovarets’ OO, Hovorun DM (2013) J Biomol Struct Dyn 31(8):913–936. doi:10.1080/07391102.2012.715041
Šponer J, Leszczynski J, Hobza P (1996) J Biomol Struct Dyn 14(1):117–135
Noorduis P, Holwerda U, van der Wilt CL, Van Croeningen CJ, Smid K, Meijer S, Pinedo HM, Peters GJ (2004) Ann Oncol 15(7):1025–1032. doi:10.1093/annonc/mdh264
Yoshiok A, Tanak S, Hiraok O, Koyam Y, Hirot Y, Ayusawa D, Seno T, Garrett C, Wataya Y (1987) J Biol Chem 262:8235–8241
Coll M, Saal D, Frederick CA, Aymami J, Rich A, Wang AHJ (1989) Nucl Acids Res 17(3):911–923. doi:10.1093/nar/17.3.911
Sowers LC, Eritja R, Kaplan B, Goodman MF, Fazakerly GV (1988) J Biol Chem 263:14794–14801
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., Wallingford CT
Hobza P, Zahradnik R (1988) Intermolecular complexes. Elsevier, Amsterdam
van Duijneveldt FB, van Duijneveldt-van de Rijdt JGCM, van van Lenthe JH (1994) Chem Rev 94:1873–1885. doi:10.1021/cr00031a007
Alkorta I, Rozas I, Elguero J (1998) Struct Chem 9:243–247. doi:10.1023/A:1022424228462
Bader RFW (1991) Chem Rev 91:893–928. doi:10.1021/cr00005a013
Iogansen V (1999) Spectrochim Acta, Part A 55:1585–1612. doi:10.1016/S1386-1425(98)00348-5
Brovarets’ OO, Hovorun DM (2013) Adasdfsadf. Phys Chem Chem Phys 15:20091–20104. doi:10.1039/c3cp52644e
Espinosa E, Molins E, Lecomte C (1998) Chem Phys Lett 285:170–173. doi:10.1016/S0009-2614(98)00036-0
Reynisson J, Steenken S (2002) Phys Chem Chem Phys 4:5353–5358. doi:10.1039/b206342e
Sharma P, Mitra A, Sharma S, Singh H (2007) J Chem Sci 119:525–531. doi:10.1007/s12039-007-0066-9
Wärmländer S, Sponer JE, Sponer J, Leijon M (2002) J Biol Chem 277:28491–28497. doi:10.1074/jbc.M202989200
Kawahara SI, Uchimaru T, Sekine M (2000) J Mol Struct 530:109–117. doi:10.1016/S0166-1280(00)00329-8
Guerra CF, Bickelhaupt FM, Snijders JG, Baerends EJ (1999) Chem Eur J 5:3581–3595
Šponer J, Leszczynski J, Hobza P (1996) J Phys Chem 100:1965–1974. doi:10.1021/jp952760f
Leszczynski J (1992) Int J Quantum Chem 44:43–55. doi:10.1002/qua.560440708
Gorb L, Podolyan Y, Dziekonski P, Sokalski WA, Leszczynski J (2004) J Am Chem Soc 126:10119–10129. doi:10.1021/ja049155n
Rosenberg JM, Seeman NC, Day RO, Rich A (1976) J Mol Biol 104:145–167. doi:10.1016/0022-2836(76)90006-1
Brovarets’ OO, Hovorun DM (2013) J Comput Chem 34:2577–2590. doi:10.1002/jcc.23412
Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, Howell SB, Boland CR (1999) Gastroenterology 117:123–131
Acknowledgments
Supported by the Science and Technology Development Project of Pingdingshan (2012064).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, Z.M., Wang, G.L., Wang, H.L. et al. MP2 study on the hydrogen-bonding interaction between 5-fluorouracil and DNA bases: A,C,G,T. Struct Chem 25, 1465–1474 (2014). https://doi.org/10.1007/s11224-014-0427-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0427-1