Abstract
Background
For prolactinoma patients, dopamine agonists (DAs) are indicated as the first-line treatment and surgery is an adjunctive choice. However, with the development of surgical technique and equipment, the effect of surgery has improved. The aim of this study was to assess the efficacy and safety of surgery versus DAs in patients with different types of prolactinomas.
Methods
A systematic search of literature using Web of Science, PubMed, Cochrane Library, and Clinical Trial databases was conducted until July 12, 2019. Prolactinoma patients treated with DAs (bromocriptine or cabergoline) or surgery (microscopic or endoscopic surgery) were included. Outcomes included the biochemical cure rate, recurrence rate, prolactin level, improvement rates of symptoms, and incidence rates of complications. A random-effects model was used to pool the extracted data. Qualitative comparisons were conducted instead of quantitative comparison.
Results
DAs were better than surgery in terms of the biochemical cure rate (0.78 versus 0.66), but surgery had a much lower recurrence rate (0.19 versus 0.57). Full advantages were not demonstrated in improvement rates of symptoms and incidence rates of complications with both treatment options. In microprolactinoma patients, the biochemical cure rate of endoscopic surgery was equal to the average cure rate of DAs (0.86 versus 0.86) and it surpassed the biochemical cure rate of bromocriptine (0.86 versus 0.76). In macroprolactinoma patients, endoscopic surgery was slightly higher than bromocriptine (0.66 versus 0.64) in terms of the biochemical cure rate.
Conclusion
For patients with clear indications or contraindications for surgery, choosing surgery or DAs accordingly is unequivocal. However, for patients with clinical equipoise, such as surgery, especially endoscopic surgery, in microprolactinoma and macroprolactinoma patients, we suggest that neurosurgeons and endocrinologists conduct high-quality clinical trials to address the clinical equipoise quantitatively.
Similar content being viewed by others
Background
Prolactinomas are the most common type of hormone-secreting pituitary tumors and they represent 40% of all pituitary tumors [1]. Dopamine agonists (DAs), including bromocriptine and cabergoline, are recommended as the first-line treatment for most prolactinomas. Surgery is only an adjunctive choice when resistance or intolerance to DAs occurs or severe complications, such as pituitary apoplexy or cerebrospinal fluid leak, develop [2].
However, with the development of surgical technique and equipment, especially endoscopic surgery, it is time to reassess the relationship between DAs and surgery. Only few retrospective studies [3,4,5,6,7,8] have compared the efficacy and safety between surgery and DAs in some specific subgroups of prolactinoma patients. And few meta-analyses discussed the difference among treatments for prolactinoma in some outcomes, mostly remission rates and recurrence rates [9,10,11]. As far as we know, no meta-analysis discussed comprehensive efficacy (remission and symptom relief) and safety (relapse and complications) for various treatments of a full spectrum of prolactinoma patients. Because of the lack of a large sample-sized study comparing these two methods in all prolactinoma patients, we conducted this meta-analysis to compare the efficacy and safety of surgery versus DAs in all prolactinoma patients with a focus on the following outcomes: biochemical cure rate, recurrence rate, symptom improvement rates, and incidence rates of complications.
Methods
This study was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) [12].
Literature research
Web of Science, PubMed, Cochrane Library, and Clinical Trial databases were independently searched until September 3, 2019, by Cai and Zhu. Search strategy combined MESH terms including “Prolactinoma,” “Dopamine Agonists,” “Microscopy,” and “Endoscopy” with free-text words including “Microprolactinoma,” “Macroprolactinoma,” “Giant prolactinoma,” “Bromocriptine,” “Cabergoline,” and “Surgery” (Supplementary file 1). Studies were restricted to the English language in this research.
Inclusion criteria
The eligibility criteria consisted of the following items: (1) only studies that included patients who had been diagnosed with prolactinoma. Prolactinomas are classified by the size of the tumor as microprolactinoma (< 10 mm), macroprolactinoma (≥ 10 mm), and giant prolactinoma (> 40 mm) [13]; (2) required treatments included surgery (microscopic surgery or endoscopic surgery) or DAs (bromocriptine or cabergoline). Patients in the DAs group only received DAs, but patients in the surgery group may have received DAs before surgery; (3) included studies reported the data of at least one available outcome that was assessed in this study.
Exclusion criteria
We excluded the following studies: (1) papers that assessed other pituitary tumors; (2) studies that utilized other DAs, gamma knife surgery, or radiation therapy; (3) studies that included less than 10 patients.
Extraction of data
Following data were extracted from each paper: author, year of publication, subtype of prolactinoma, intervention, size of sample, gender proportion, mean age, and mean follow-up duration. We also assessed the biochemical cure rate, recurrence rate, and the following variables before and after treatment: prolactin level, visual impairment, headache, menstrual disturbance, galactorrhoea, adrenocorticotropic hormone (ACTH) insufficiency, thyroid-stimulating hormone (TSH) deficiency, hypopituitarism (one or more deficiencies), and diabetes insipidus. Recurrence was defined as the observation of hyperprolactinemia after a period of normalization after surgery and withdrawal of DAs. The assessment of hormonal deficiencies was performed by calculating the presence of hormonal deficiencies after treatment. The extraction of data was independently carried out by Cai and Zhu.
Quality assessment
The same two reviewers (Cai and Zhu) assessed risk of bias for included studies independently. ROB 2 Cochrane risk of bias tool was used for the randomized controlled trials (RCTs) and ROBINS-I tool for non-randomized controlled trials (non-RCTs) [14, 15]. As no available text-book quality guidelines for case-series studies, we used a tool developed by Moga et al. to assess case-series studies [16]. No cutoff scores were provided within this tool, so we gave one point to each “yes” answer and zero to each “no” and “unclear” answer.
Statistical analysis
To conduct a meta-analysis of single rates, STATA Version 12.0 and MetaAnalyst Beta 3.13 were applied separately for assessing the biochemical cure rate, recurrence rate, and other parameters. A RE (random-effects) model using Mantel-Haenszel heterogeneity method was also used in these two programs. RevMan Version 5.0 was used to evaluate the pooled mean difference between pre- and post-treatment prolactin levels using the RE model. With this procedure, I-squared values were calculated to assess the heterogeneity of pooled results. Subgroup analysis and meta-regression analysis of mean age, gender, publication year, subtypes of prolactinoma, subtypes of surgery, and drug species were conducted to discover the sources of heterogeneity. A funnel plot was used to evaluate the publication bias. As the indications for surgery and DAs were significantly different from each other, we only conducted qualitative comparison instead of formal quantitative comparison in the meta-analysis.
Results
Included studies
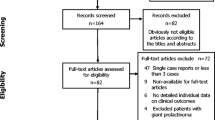
Based on our search strategy, 4373 papers were identified in the databases. From these 4373 papers, 4174 papers were excluded after screening the titles and abstracts (Fig. 1). The remaining 199 full-text articles were assessed for eligibility. During this process, 53 articles were excluded because of differences in the population, interventions, outcomes, or type of articles compared with inclusion criteria.
Finally, a total of 146 articles were included in this meta-analysis. Further, 82 of these 146 articles provided data for the DAs group [3,4,5,6,7,8, 13, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91] and 72 articles provided data for the surgery group [3,4,5,6,7,8, 13, 68, 92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. Details of these 146 studies are presented in Table 1 and Supplementary Tables 1 and 2 separately. The meta-analysis included 9007 patients with no restriction on age and gender. Most studies reported the biochemical cure rates after treatment, but the recurrence rates were provided only in most studies on surgery and few studies on DAs focusing on withdrawal of medicine.
Quality assessments showed some concern for most RCTs because of their unclear description about random process and prespecified analysis plan. The assessments also found 18.8% (6/32) high, 21.9% (7/32) moderate, and 59.4% (19/32) low overall bias for non-RCTs, and the main bias was confounding and excluding patients due to missing data. The average score for case series studies was 11.9 [4,5,6,7,8,9,10,11,12,13,14,15,16], and the main bias came from study design (Q2–4) and unclear description of statistical analysis (Q14). The summary of risk of bias within studies was provided in Supplementary Fig. 1 and Supplementary Tables 3, 4 and 5.
Biochemical cure rate
A total of 81 studies [4,5,6,7,8, 13, 68, 84, 92,93,94,95,96,97, 99,100,101,102,103,104,105,106,107,108,109,110,111,112, 114, 118, 120,121,122,123, 125, 127,128,129,130,131,132,133, 135,136,137, 139, 141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156] comprising 4397 patients who received surgery and 74 studies [3,4,5,6, 8, 13, 17,18,19,20,21, 25, 26, 28,29,30,31,32,33,34,35,36, 38, 42,43,44,45,46, 48,49,50,51, 54,55,56,57,58, 60, 61, 65,66,67,68,69,70,71,72,73, 76, 79,80,81, 85,86,87, 89, 91] comprising 2659 patients who used DAs were included in this part of the research. The pooled prolactin normalization rates were 0.66 (0.62, 0.71) (I2 = 93.8%, p = 0.000) in the surgery group and 0.78 (0.75, 0.82) (I2 = 89.4%, p = 0.000) in the DAs group, respectively (Fig. 2). Because of high heterogeneity, subgroup analysis and meta-regression analysis were conducted to detect the source of high heterogeneity. In the surgery group, although no significant decrease in heterogeneity was found in the subgroup analysis (Supplementary Fig. 2), meta-regression analysis detected that gender (p = 0.019) and macroprolactinoma (p = 0.001) were statistically significant factors causing heterogeneity. In the subgroup analysis, macroprolactinoma patients showed a lower biochemical cure rate (0.57 versus 0.66) compared with total surgery-treated patients, but in macroprolactinoma patients, the biochemical cure rate was higher (0.79 versus 0.66) than total surgery-treated patients (Supplementary Fig. 2). And regression analysis identified that female patients showed a positive trend in the rates compared with male patients. Because the surgery group included patients with or without DAs treatment history, we conducted subgroup analysis based on DAs treatment history to explore the normalization rate of surgery treated population without DAs treatment history. Results showed similar normalization rates in without DAs treatment history subgroup (0.69 (0.44,0.94); I2 = 94.5%, p = 0.000) with that in the whole surgery treated population (Supplementary Fig. 8). In the DAs group, subgroup analysis was carried out based on decades, subtypes of prolactinoma, and drug species (Supplementary Fig. 2), and the giant prolactinoma (I2 = 62.3%, p = 0.010) subgroup showed a decrease in important heterogeneity (Table 2). Meta-regression analysis of the DAs group also showed that giant prolactinoma (p = 0.029) and bromocriptine (p = 0.024) were important sources of heterogeneity (Table 4), and their rates were lower than the rates in all patients (0.62 versus 0.78; 0.70 versus 0.78). The funnel plot for the surgery group (Supplementary Fig. 3A) showed a symmetric distribution on either side of the middle line, but an asymmetric distribution for the DAs group. Based on the funnel plot, some degree of publication bias was found in the DAs group (Supplementary Fig. 3B).
Cumulative meta-analysis was also conducted to detect the changes in the biochemical cure rate over time. Results showed an overall increasing trend of the biochemical cure rate of surgery, and after the year 2000, the biochemical cure rate of endoscopic surgery was consistently higher than that of bromocriptine (Fig. 4A).
Recurrence rate
This part consisted of 36 studies [4, 6, 93, 100, 102, 105, 111, 112, 114, 116, 120,121,122, 125, 127, 128, 132, 135, 138, 139, 141, 142, 145, 146, 148, 150, 154,155,156] comprising 1215 patients who underwent surgery and 19 studies [24, 27, 34, 39, 41, 47, 59, 62, 64, 68, 75, 82, 84, 85, 87] comprising 835 patients who used DAs. The recurrence rate of surgery was 0.19 (0.15, 0.24) (I2 = 83.7%, p = 0.000) and 0.57 (0.48, 0.67) (I2 = 89.2%, p = 0.000) for DAs (Fig. 3). Because of the high heterogeneity in surgery and DAs, subgroup analysis was carried out based on decades, subtypes of prolactinoma, subtypes of surgery, and drug species (Table 3; Supplementary Fig. 4). The following significant decreases in heterogeneity were detected: 2000–2009 (I2 = 47.1%, p = 0.093), microprolactinoma (I2 = 65.6%, p = 0.002), microscopic surgery (I2 = 65.7%, p = 0.020), and endoscopic surgery (I2 = 0.0%, p = 0.865) for surgery and bromocriptine (I2 = 15.5%, p = 0.277) for DAs (Table 3). Meta-regression analysis did not detect any important factors with respect to heterogeneity sources (Table 4).
Cumulative meta-analysis of recurrence rates was carried out. Results showed that the recurrence rate of DAs decreased from 0.86 (0.73, 1.00) in 1991 to 0.57 (0.48, 0.67) in 2018. In the surgery group, the recurrence rate consistently reduced from 0.29 (0.15, 0.43) in 1985 to 0.18 (0.14, 0.21) in 2018 (Fig. 4B).
Prolactin level
A total of 8 studies [7, 98, 124, 134, 150] comprising 555 patients in the surgery group and 27 studies [7, 31, 33, 38, 40, 42,43,44, 46, 48, 54, 55, 59, 78, 81, 83, 84, 90] comprising 954 patients in the DAs group were included in this part of research. Based on the pooled results, the mean differences in the prolactin levels between pre- and post-treatment were 396.80 ng/ml (222.33, 571.27) (I2 = 99%, p < 0.001) for surgery and 375.26 ng/ml (316.21, 434.31) (I2 = 98%, p < 0.001) for DAs (Supplementary Fig. 5). Sensitive analysis was conducted to find the source of heterogeneity, but no notable decrease in heterogeneity was detected.
Symptom improvement rate
Improvement rate for vision impairment
In the surgery group, 114 patients from 11 studies [13, 95, 97, 124, 132, 137, 141, 143, 156] were included, and the pooled improvement rate for vision impairment was 0.68 (0.51, 0.82) (I2 = 34.8%, p = 0.018) (Table 5) with moderate heterogeneity. In the DAs group, 14 studies [5, 13, 29, 30, 33, 43, 46, 48, 71, 79] comprising 176 patients provided the required data, and the pooled improvement rate for vision impairment was 0.57 (0.38, 0.74) (I2 = 42.4%, p = 0.000) (Table 5; Supplementary Fig. 6A,7A) with moderate heterogeneity.
Headache improvement rate
A total of 3 studies [95, 98, 132] comprising 95 patients treated with surgery were included, and the pooled headache improvement rate was 0.80 (0.32, 0.97) (I2 = 46.9%, p = 0.000). Meta-analysis of this part was conducted for DAs using 35 patients from 4 studies [5, 30, 32, 46]. The pooled headache improvement rate of DAs was 0.86 (0.72, 0.94) (I2 = 0%, p = 0.416) with low heterogeneity (Table 5; Supplementary Fig. 6B,7B).
Improvement rate for menstrual disturbance
A total of 3 studies [94, 141, 154] comprising 226 patients treated with surgery and 6 studies [20, 28, 30, 71] comprising 123 patients who used DAs were included, and the pooled improvement rates for menstrual disturbance were 0.68 (0.62, 0.74) (I2 = 0%, p = 0.327) and 0.71 (0.16, 1.00) (I2 = 47.5%, p = 0.000), respectively (Table 5; Supplementary Fig. 6C,7C).
Galactorrhoea improvement rate
This research included 3 studies [124, 132, 141] comprising 176 patients treated with surgery and 6 studies [30, 32, 43, 71] comprising 29 patients who used DAs to assess the galactorrhoea improvement rate after these treatments. The pooled galactorrhoea improvement rates were 0.33 (0.01, 0.94) (I2 = 47.1%, p = 0.000) after surgery and 0.89 (0.72, 0.96) (I2 = 0%, p = 0.493) after DAs, respectively (Table 5; Supplementary Fig. 6D,7D).
Complications
Incidence rate of ACTH insufficiency
A total of 387 patients from 11 studies [3, 5, 6, 13, 93, 98, 121, 151, 152, 154] that applied surgery and 286 patients from 9 studies [3, 5, 13, 33, 45, 73, 78] that utilized DAs were included, and the pooled incidence rates of ACTH insufficiency were 0.25 (0.13, 0.43) (I2 = 46.7%, p = 0.000) for surgery and 0.10 (0.06, 0.16) (I2 = 26.0%, p = 0.121) for DAs, respectively (Table 5; Supplementary Fig. 6E,7E).
Incidence rate of TSH deficiency
In this part, 12 studies [3,4,5,6, 13, 93, 98, 151, 152, 154] comprising 475 patients who underwent surgery and 7 studies [3, 5, 13, 23, 61, 73, 88] comprising 194 DAs-treated patients were included, and the pooled estimated rates were 0.24 (0.14, 0.38) (I2 = 45.4%, p = 0.000) and 0.19 (0.12, 0.28) (I2 = 26.4%, p = 0.134) after surgery and DAs, respectively (Table 5; Supplementary Fig. 6F,7F).
Incidence rate of hypopituitarism
A total of 709 surgery-treated patients from 11 studies [5, 6, 97, 124, 141, 147, 148, 156] and 99 DAs-treated patients from 4 studies [5, 48] were included to assess the incidence rate of hypopituitarism. The pooled incidence rates were 0.17 (0.06, 0.38) (I2 = 48.4%, p = 0.000) for surgery and 0.29 (0.13, 0.54) (I2 = 41.6%, p = 0.015) for DAs, respectively (Table 5; Supplementary Fig. 6G,7G).
Incidence rate of diabetes insipidus
Because of the lack of studies that used DAs and reported the incidence rate of diabetes insipidus, only 1616 surgery-treated patients from 27 studies [3,4,5, 93, 98, 99, 115, 117, 124, 126, 132, 138, 140, 141, 143, 145, 147,148,149,150,151,152,153,154, 156] were included to detect the pooled incidence rate. The estimated incidence rate of diabetes insipidus after surgery was 0.17 (0.12, 0.25) (I2 = 47.1%, p = 0.000) (Table 5; Supplementary Fig. 6H).
Discussion
DAs are the preferred choice in the current guideline, and they are used for treating symptomatic microprolactinomas and macroprolactinomas [157]. Compared with DAs, surgery has very limited indications, which include the following: (1) intolerance or resistance to DAs; (2) acute complications such as pituitary apoplexy and cerebrospinal fluid leak [157]. Some new indications have been discussed in other papers, which include the following: (3) Young patients with high complete resection rate; (4) unwillingness to take long-term medication; (5) cystic prolactinoma; (6) partial resistance to treatment; and (7) requirement of high dose of cabergoline [158]. The reasons for these limited indications are a reported high recurrence rate (7–50%), possible complications, and requirement of experienced neurosurgeons [157].
Over the past 5 decades, the endoscope has developed from a diagnostic tool to a mature surgical technique with concepts of minimally invasive surgery and key-hole surgery [159]. An increasing number of neurosurgeons have accepted this vivifying technique and have promoted its indications. Based on our results, surgery, especially endoscopic surgery, has already shown satisfactory efficacy and safety in some subgroups of prolactinoma patients, and it is time to re-evaluate the surgical indications of prolactinoma.
DAs versus surgery for microprolactinoma
Symptomatic microprolactinoma patients are recommended to receive DAs in the current guideline [157], although a microprolactinoma rarely grows. But the pooled estimated biochemical cure rate of endoscopic surgery was the same as that of DAs (0.86 versus 0.86) and it was slightly higher than that of bromocriptine (0.86 versus 0.76). Furthermore, the recurrence rates of surgery, both microscopic and endoscopic surgery, were much lower than those of DAs (0.10 versus 0.63). In another meta-analysis conducted by Ma et al. [10], the reported long-term remission rates for microprolactinoma were 56% (medication) versus 91% (surgery). The difference between their results and our results may have arisen from different inclusion criteria, as they excluded patients utilizing DAs before surgery. Zamanipoor et al. also conducted a meta-analysis and found the long-term remission rates were 36% versus 83% for medication and surgery separately(9). This may be due to that they only include patients with medicine withdrawal. It is notable that some countries like China do not allow the use of cabergoline, and patients living in such countries may consider surgery to be a better choice than bromocriptine.
DAs versus surgery for macroprolactinoma
All macroprolactinoma patients with or without symptoms are recommended to use DAs [157]. The same preference was detected in our results, which showed that DAs had a higher biochemical cure rate than surgery (0.77 versus 0.57). However, some interesting results were also found in the subgroup analysis. The only one included microscopic study in the microsurgery group reported the highest biochemical cure rate. Furthermore, endoscopic surgery and bromocriptine were at the same level in terms of the biochemical cure rate (0.66 versus 0.64) and endoscopic surgery was lower than bromocriptine in terms of the recurrence rate (0.11 versus 0.92). Results for the long-term remission rates in the study by Ma et al. [10] showed a similar tendency to that in our study (77% versus 44%). But the results from Zamanipoor et al. showed that the long-term remission rates were 28% versus 60% for medication and surgery separately [9]. The difference between their results and ours may come from that they only include patients with medication withdrawal.
DAs versus surgery for giant prolactinoma
For giant prolactinoma, we failed to include studies reporting the biochemical cure rate after microscopic surgery or bromocriptine and the recurrence rate after any treatment. This may be because of our strict inclusion criteria, as we excluded studies with less than 10 patients or studies using another treatment like radiotherapy. In our results, DAs showed a higher biochemical cure rate than surgery (0.62 versus 0.35). Similar but exaggerated results were reported by Lv et al. [13] (0.48 versus 0, DAs versus surgery). Hamidi et al. also detected similar remission rates (58.8% versus 53.6%, DAs versus surgery). Because of the lack of data from giant prolactinoma patients, no recommendations are found in the current guidelines. Further researches should address this question and verify our results in future guidelines.
Comparison of relief of symptoms between DAs and surgery
A large prolactinoma can compress the surrounding structures and can cause severe vision impairment and headache [160], which are also the indications for surgery. Lv et al. [13] reported that DAs and surgery had a similar recovery rate for visual impairment. However, it is interesting that the current research reported a slightly higher improvement rate for vision impairment in surgery-treated patients (0.68 versus 0.57) and a comparable headache improvement rate in DAs-treated patients (0.80 versus 0.86); thus, showing that surgery and DAs may have a similar ability in relieving nerve compression.
We found preference of DAs in terms of the improvement rate for menstrual disturbance (0.71 versus 0.68) and galactorrhea (0.89 versus 0.33). Nayan et al. [11] conducted a meta-analysis on the fertility after surgery in prolactinoma patients, and they reported a significant decrease in the pooled prevalence of galactorrhea from 84 to 29%. The reduction was greater than that in our study, which may have been caused by gender restriction in the inclusion criteria.
Comparison of the rate of complications between DAs and surgery
A low rate of complications was noted for both treatments. Our results revealed a preference for DAs in ACTH insufficiency (0.10 versus 0.25) and TSH deficiency (0.19 versus 0.24) but a higher incidence rate of hypopituitarism (0.29 versus 0.17) after DAs. Oksana et al. [5] reported similar results in ACTH insufficiency and TSH deficiency but a contrary result in hypopituitarism, and all of the results from their study were higher than our results (ranging from 27 to 69%). A different population, as they only included giant prolactinoma cases, may explain this discrepancy.
The incidences of diabetes insipidus in different studies range from 2.5 to 100%, with the pooled result being 0.174 (0.118, 0.251). Because no studies on DAs-treated patients with diabetes insipidus were included, we failed to compare the outcome between DAs and surgery.
Comparison of the cost of therapy between DAs and surgery
The cost of DAs and surgery is a complex consideration, and contrary results have been reported. Lian et al. [161] reported that for microprolactinoma patients, the estimated costs of surgery and DAs were ¥22,527 and ¥20,555. For macroprolactinoma patients, the estimated costs were ¥42,357/¥44,094 in males/females for surgery and ¥31,461/¥27,178 in males/females for DAs. Similar results were found by Zhen et al. [162]. But Corinna et al. [163] reported different results; they reported that the lifetime costs of surgery, bromocriptine, and cabergoline were $40,473, $41,601, and $70,696, respectively. Further studies are needed to determine which method is more cost-effective.
DAs treatment before surgery?
In the current research, we conducted subgroup analysis for surgery treated population based on DAs treatment history and found similar normalization rates between patients with DAs treatment history (0.66) and without DAs treatment history (0.69; Supplementary Fig. 8). This result showed that DAs treatment before surgery may not influence the efficiency of surgery. Because all included researches for the safety analysis only discussed patients with DAs treatment history or provided inseparable data of these two situations, we did not explore the difference of surgery safety between patients with or without DAs treatment history.
Duration of medication
The mean duration of medication treatment in the DAs treatment group was 44.5 months. But most studies defined resistance to DA as a lack of PRL normalization and a failure to decrease tumor size despite an adequate dose of DA treatment for 3 or 6 months [99, 127]. For patients who were resistant to DAs treatment, they were recommended to increase the dose to maximal tolerable doses [157]. And for patients who have no response to DAs, they were recommended to accept transsphenoidal surgery [157].
Advantages and limitations
As this was the first study to compare the efficacy and safety between DAs and surgery in patients with all types of prolactinomas, we included a large sample size of up to 6162 patients.
The major limitation of the present research was that we could not perform a two-arm meta-analysis due to the lack of prospective randomized controlled trials. We could only collect the data from single-arm studies. And because of the different indications for surgery and DAs, the patient groups differed significantly between each other. So, we conducted qualitative comparison between treatments instead of a quantitative comparison in the current meta-analysis. Randomized controlled trials of DAs and surgery are expected in the future.
Another limitation was the high heterogeneity of the biochemical cure rate and the recurrence rate. Although we conducted a subgroup analysis and a meta-regression analysis to identify the source of heterogeneity, we only found that giant prolactinoma and bromocriptine could partially explain the heterogeneity. We failed to collect the following data and proceed with a comparison of the following parts: biochemical cure rate in giant prolactinoma patients using microscopic surgery or bromocriptine, recurrence rate in all giant prolactinoma patients, recurrence rate in microprolactinoma patients treated with bromocriptine, and incidence rate of diabetes insipidus in DAs-treated patients. The lack of data may have arisen from our inclusion criteria of patient size limitation. Most DAs withdrawal studies focused on cabergoline, and few studies on bromocriptine were excluded from this research because of our exclusion criteria. Further clinical researches on these patients are needed.
The present study did not include the radiological parameters of prolactinoma. Further researches are needed to verify our results.
Conclusion
The present meta-analysis serves as the first study to compare the efficacy and safety between DAs and surgery in microprolactinoma and macroprolactinoma patients. We concluded that for patients with clear indications or contraindications for surgery, choosing surgery or DAs accordingly is unequivocal. However, for patients with clinical equipoise, further controlled clinical trials are expected to address it. In this meta-analysis, we discovered that surgery, especially endoscopic surgery, showed comparable efficacy and safety in microprolactinoma and macroprolactinoma patients with a considerable biochemical cure rate, lower recurrence rate, and similar improvement rates of symptoms and incidence rates of complications. With the development of surgical technique and equipment, the efficacy and safety of surgery have greatly improved. Therefore, we suggest that neurosurgeons and endocrinologists conduct high-quality clinical trials to address the clinical equipoise quantitatively.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- TSH:
-
Thyroid-stimulating hormone
- DAs:
-
Dopamine agonists
- RE:
-
Random-effects
- CAB:
-
Cabergoline
- BRC:
-
Bromocriptine
- NA:
-
Not applicable
References
Wong A, Eloy JA, Couldwell WT, Liu JK. Update on prolactinomas. Part 1: Clinical manifestations and diagnostic challenges. J Clin Neurosci. 2015;22:1562–7.
Faje A, Nachtigall L. Current treatment options for hyperprolactinemia. Expert Opin Pharmacother. 2013;14:1611–25.
Berezin M, Shimon I, Hadani M. Prolactinoma in 53 men: Clinical characteristics and modes of treatment (male prolactinoma). J Endocrinol Invest. 1995;18:436–41.
Samaan NA, Schultz PN, Leavens TA, Leavens ME, Lee YY. Pregnancy after treatment in patients with prolactinoma: Operation versus bromocriptine. Am J Obstet Gynecol. 1986;155:1300–5.
Hamidi O, Van Gompel J, Gruber L, Kittah NE, Donegan D, Philbrick KA, et al. Management and outcomes of giant prolactinoma: a series of 71 patients. Endocr Pract. 2019;25:340–52.
Hong JW, Lee MK, Kim SH, Lee EJ. Discrimination of prolactinoma from hyperprolactinemic non-functioning adenoma. Endocrine. 2010;37:140–7.
Asano S, Ueki K, Suzuki I, Kirino T. Clinical features and medical treatment of male prolactinomas. Acta Neurochir (Wien). 2001;143:465–70.
Andereggen L, Frey J, Andres RH, El-Koussy M, Beck J, Seiler RW, et al. 10-year follow-up study comparing primary medical vs. surgical therapy in women with prolactinomas. Endocrine. 2017;55:223–30.
Zamanipoor Najafabadi AH, Zandbergen IM, de Vries F, Broersen LHA, van den Akker-van Marle ME, Pereira AM, et al. Surgery as a Viable Alternative First-Line Treatment for Prolactinoma Patients. A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2020;105.
Ma Q, Su J, Li Y, Wang J, Long W, Luo M, et al. The chance of permanent cure for micro- and macroprolactinomas, medication or surgery? A systematic review and meta-analysis. Front Endocrinol. 2018;9:1–10.
Lamba N, Noormohamed N, Simjian T, Alsheikhb MY, Jamalb A, Doucetteb J, et al. Fertility after transsphenoidal surgery in patients with prolactinomas: A meta-analysis. Clin Neurol Neurosurg. 2019;176:53–60.
Hutton B, Wolfe D, Moher D, Shamseer L. Reporting guidance considerations from a statistical perspective: overview of tools to enhance the rigour of reporting of randomised trials and systematic reviews. Evid Based Ment Health. 2017;20:46–52.
Lv L, Hu Y, Yin S, Zhou P, Yang Y, Ma W, et al. Giant prolactinomas: Outcomes of multimodal treatments for 42 cases with long-term follow-up. Exp Clin Endocrinol Diabetes. 2019;127:295.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. 2016;69(199-207):e2.
Zarate A, Canales ES, Alger M, Forsbach G. The effect of pregnancy and lactation on pituitary prolactin-secreting tumours. Acta Endocrinol (Copenh). 1979;92:407–12.
Coculescu M, Simionescu N, Oprescu M, D. A. Bromocriptine treatment of pituitary adenomas. Evaluation of withdrawal effect. Endocrinologie. 1983;21:157–68.
Nissim M, Ambrosi B, Bernasconi V, Giannattasio G, Giovanelli MA, Bassetti M, et al. Bromocriptine treatment of macroprolactinomas: studies on the time course of tumor shrinkage and morphology. J Endocrinol Invest. 1982;5:409–15.
Archer DF, Lattanzi DR, Moore EE, Harger JH, Herbert DL. Bromocriptine treatment of women with suspected pituitary prolactin-secreting microadenomas. Am J Obstet Gynecol. 1982;143:620.
Hancock KW, Scott JS, Lamb JT, Gibson RM, Chapman C. Conservative management of pituitary prolactinomas ; evldence for bromocriptine-induced regression. Br J Obstet Gynaecol. 1980;87:523–9.
Hildebrandt G, Bauer T, Stracke H, Fassbender WJ, Mueller HW, Agnoli AL, et al. Surgery, dopamine agonist therapy of combined treatment--results in prolactinoma patients after a 12 month follow-up. Zentralbl Neurochir. 1992;53:123–34.
Beckers A, Petrossians P, Abs R, Flandroy P, Stadnik T, De Longueville M, et al. Treatment of macroprolactinomas with the long-acting and repeatable form of bromocriptine: a report on 29 cases. J Clin Endocrinol Metab. 1992;75:275–80.
van T, Verlaat JW, Croughs RJ. Withdrawal of bromocriptine after long-term therapy for macroprolactinomas; effect on plasma prolactin and tumour size. Clin Endocrinol (Oxf). 1991;34:175–8.
Verlaat JWV, Croughs RJM, Hendriks MJ, Bosma NJ. Results of primary treatment with bromocriptine of prolactinomas with extrasellar extension. Can J Neurol Sci. 1990;17:71–3.
Hildebrandt G, Zierski J, Christophis P, Laun A, Schatz H, Lancranjan I, et al. Rhinorrhea following dopamine agonist therapy of invasive macroprolaetinoma. Acta Neurochir (Wien). 1989;96:107–13.
Wang C, Lam KSL, Ma JTC, Chan T, Liu MY, Yeung RTT. Long-term treatment of hyperprolactinaemia with bromocriptine: effect of drug withdrawal. Clin Endocrinol (Oxf). 1987;27:363–71.
Ferrari C, Paracchi A, Mattei AM, de Vincentiis S, D'Alberton A, Crosignani P. Cabergoline in the long-term therapy of hyperprolactinemic disorders. Acta Endocrinol (Copenh). 1992;126:489–94.
Sarno AD, Landi ML, Cappabianca P, Salle FD, Rossi FW, Pivonello R, et al. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: Prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86:5256–61.
Colao A, Di Sarno A, Landi ML, Scavuzzo F, Cappabianca P, Pivonello R, et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: A prospective study in 110 patients. J Clin Endocrinol Metab. 2000;85:2247–52.
Cannavò S, Curtò L, Squadrito S, Almoto B, Vieni A, Trimarchi F. Cabergoline: A first-choice treatment in patients with previously untreated prolactin-secreting pituitary adenoma. J Endocrinol Invest. 1999;22:354–9.
Colao A, Di Sarno A, Sarnacchiaro F, Ferone D, Di Renzo G, Merola B, et al. Prolactinomas resistant to standard dopamine agonists respond to chronic cabergoline treatment. J Clin Endocrinol Metab. 1997;82:876–83.
Ferrari CI, Abs R, Bevan JS, Barbant G, Ciccarelli E, Motta T, et al. Treatment of macroprolactinoma with cabergoline: a study of 85 patients. Clin Endocrinol (Oxf). 1997;46:409–13.
Muratori M, Arosio M, Gambino G, Romano C, Biella O, Faglia G. Use of cabergoline in the long-term treatment of hyperprolactinemic and acromegalic patients. J Endocrinol Invest. 1997;20:537–46.
Delgrange EDM, Donckier J. Effects of the dopamine agonist cabergoline in patients with prolactinoma intolerant or resistant to bromocriptine. Eur J Endocrinol. 1996;134:454–6.
Sabuncu T, Arikan E, Tasan E, Hatemi H. Comparison of the effects of cabergoline and bromocriptine on prolactin levels in hyperprolactinemic patients. Intern Med. 2001;40:857–61.
Naliato ECO, Violante AHD, Caldas D, Filho AL, Loureiro CR, Fontes R, et al. Body fat in nonobese women with prolactinoma treated with dopamine agonists. Clin Endocrinol (Oxf). 2007;67:845–52.
Shimon I, Benbassat C, Hadani M. Effectiveness of long-term cabergoline treatment for giant prolactinoma: study of 12 men. Eur J Endocrinol. 2007;156:225–31.
Colao A, Di Sarno A, Guerra E, Pivonello R, Cappabianca P, Caranci F, et al. Predictors of remission of hyperprolactinaemia after long-term withdrawal of cabergoline therapy. Clin Endocrinol (Oxf). 2007;67:426–33.
Chattopadhyay A, Bhansali A, Masoodi SR. Long-term efficacy of bromocriptine in macroprolactinomas and giant prolactinomas in men. Pituitary. 2005;8:147–54.
Biswas M, Smith J, Jadon D, McEwan P, Rees DA, Evans LM, et al. Long-term remission following withdrawal of dopamine agonist therapy in subjects with microprolactinomas. Clin Endocrinol (Oxf). 2005;63:26–31.
Colao A, Vitale G, Di Sarno A, Spiezia S, Guerra E, Ciccarelli A, et al. Prolactin and prostate hypertrophy: A pilot observational, prospective, case-control study in men with prolactinoma. J Clin Endocrinol Metab. 2004;89:2770–5.
Corsello SM, Ubertini G, Altomare M, Lovicu RM, Migneco MG, Rota CA, et al. Giant prolactinomas in men: efficacy of cabergoline treatment. Clin Endocrinol (Oxf). 2003;58:662–70.
Essas O, Bouguerra R, Hamzaoui J, Marrakchi Z, Hadjri S, Chamakhi S, et al. Efficacy and safety of bromocriptine in the treatment of macroprolactinomas. Annales d'Endocrinologie. 2002;63:524–31.
Dos Santos Silva CM, Barbosa FRP, Lima GAB, Warszawski L, Fontes R, Domingues RC, et al. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity. 2010;19:800–5.
Bhansali A, Walia R, Dutta P, Khandelwal N, Sialy R, Bhadada S. Efficacy of cabergoline on rapid escalation of dose in men with macroprolactinomas. Indian J Med Res. 2010;131:530–5.
Huda MSB, Athauda NB, Teh MM, Carroll PV, Powrie JK. Factors determining the remission of microprolactinomas after dopamine agonist withdrawal. Clin Endocrinol (Oxf). 2010;72:507–11.
Acharya S, Gopal R, Menon P, Bandgar TR, Shah NS. Giant prolactinoma and effectiveness of medical management. Endocr Pract. 2010;16:42–6.
Ono M, Miki N, Amano K, Kawamata T, Seki T, Makino R, et al. Individualized high-dose cabergoline therapy for hyperprolactinemic infertility in women with micro- and macroprolactinomas. Obstet Gynecol Surv. 2010;65:702–4.
Delgrange E, Daems T, Verhelst J, Abs R, Maiter D. Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: a study in 122 patients. Eur J Endocrinol. 2009;160:747–52.
Acharya SV, Gopal RA, Bandgar TR, Joshi SR, Menon PS, Shah NS. Clinical profile and long term follow up of children and adolescents with prolactinomas. Pituitary. 2009;12:186–9.
Cho E, Lee SA, Chung JY, Koh EH, Cho YH, Kim JH, et al. Efficacy and safety of cabergoline as first line treatment for invasive giant prolactinoma. J Korean Med Sci. 2009;24:874.
Naliato ECDO, Violante AHD, Caldas D, Farias MLF, Bussade I, Filho AL, et al. Bone density in women with prolactinoma treated with dopamine agonists. Pituitary. 2008;11:21–8.
Auriemma RS, Galdiero M, Vitale P, Granieri L, Lo Calzo F, Salzano C, et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology. 2013;98:299–310.
Ciresi A, Amato MC, Guarnotta V, Lo Castro F, Giordano C. Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clin Endocrinol (Oxf). 2013;79:845–52.
Martin DSYL, Andia MV, Jara AA. Long-term evolution and outcomes of microprolactinoma with medical treatment. Endocrinol Nutr. 2013;60:489–94.
Kallestrup M, Kasch H, Østerby T, Nielsen E, Jensen TS, Jørgensen JOL. Prolactinoma-associated headache and dopamine agonist treatment. Cephalalgia. 2013;34:493–502.
Rastogi A, Walia R, Dutta P, Bhansali A. Efficacy and safety of rapid escalation of cabergoline in comparison to conventional regimen for macroprolactinoma: A prospective, randomized trial. Indian J Endocrinol Metab. 2012;16:S294–6.
Anagnostis P, Adamidou F, Polyzos SA, Efstathiadou Z, Karathanassi E, Kita M. Long term follow-up of patients with prolactinomas and outcome of dopamine agonist withdrawal: a single center experience. Pituitary. 2012;15:25–9.
Yang MS, Hong JW, Lee SK, Lee EJ, Kim SH. Clinical management and outcome of 36 invasive prolactinomas treated with dopamine agonist. J Neurooncol. 2011;104:195–204.
Berinder K, Nyström T, Höybye C, Hall K, Hulting A-L. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary. 2011;14:199–207.
Barber TM, Kenkre J, Garnett C, Scott RV, Byrne JV, Wass JAH. Recurrence of hyperprolactinaemia following discontinuation of dopamine agonist therapy in patients with prolactinoma occurs commonly especially in macroprolactinoma. Clin Endocrinol (Oxf). 2011;75:819–24.
Nishio H, Fujii T, Kameyama K. Abdominal radical trachelectomy as a fertility-sparing procedure in women with early stage cervical cancer in a series of 61 women. Obstet Gynecol Surv. 2010;65:19–20.
Aycicek Dogan B, Arduc A, Tuna MM, Nasıroğlu NI, Işık S, Berker D, et al. Evaluation of atherosclerosis after cessation of cabergoline therapy in patients with prolactinoma. Anatol J Cardiol. 2015;16:440–7.
Almalki MH, Buhary B, Alzahrani S, Alshahrani F, Alsherbeni S, Alhowsawi G, et al. Giant prolactinomas: clinical manifestations and outcomes of 16 Arab cases. Pituitary. 2015;18:405–9.
Pala NA, Laway BA, Misgar RA, Dar RA. Metabolic abnormalities in patients with prolactinoma: response to treatment with cabergoline. Diabetology Metab Syndr. 2015;7:1–6.
Tirosh A, Benbassat C, Shimon I. Short-term decline in prolactin concentrations can predict future prolactin normalization, tumor shrinkage, and time to remission in men with macroprolactinomas. Endocr Pract. 2015;21:1240–7.
Kruljac I, Kirigin LS, Strinović M, Marinkovic J, PeTina HI, Herina V, et al. Treatment of prolactinomas in low-income countries. Int J Endocrinol. 2015;0:1–5.
Lee Y, Ku CR, Kim E, Hong JW, Lee EJ, Kim SH. Early prediction of long-term response to cabergoline in patients with macroprolactinomas. Endocrinol Metab. 2014;29:280.
Barbosa FRP, Dos Santos Silva CM, Lima GAB, Warszawski L, Domingues RC, Dominic M, et al. Prevalence of obstructive sleep apnea in patients with prolactinoma before and after treatment with dopamine agonists. Pituitary. 2014;17:441–9.
Rastogi A, Bhansali A, Dutta P, Singh P, Vijaivergiya R, Gupta V, et al. A comparison between intensive and conventional cabergoline treatment of newly diagnosed patients with macroprolactinoma. Clin Endocrinol (Oxf). 2013;79:409–15.
Cho KR, Jo K, Shin HJ. Bromocriptine therapy for the treatment of invasive prolactinoma: The single institute experience. Brain Tumor Res Treat. 2013;1:71–7.
Karavitaki N, Dobrescu R, Byrne JV, Grossman AB, Wass JAH. Does hypopituitarism recover when macroprolactinomas are treated with cabergoline? Clin Endocrinol (Oxf). 2013;79:217–23.
Araujo B, Belo S, Carvalho D. Pregnancy and tumor outcomes in women with prolactinoma. Exp Clin Endocrinol Diabetes. 2017;125:642.
Teixeira M, Souteiro P, Carvalho D. Prolactinoma management: predictors of remission and recurrence after dopamine agonists withdrawal. Pituitary. 2017;20:464–70.
Santharam S, Tampourlou M, Arlt W, Ayuk J, Gittoes N, Toogood A, et al. Prolactinomas diagnosed in the postmenopausal period: Clinical phenotype and outcomes. Clin Endocrinol (Oxf). 2017;87:508–14.
Schwetz V, Librizzi R, Trummer C, Theiler G, Stiegler C, Pieber TR, et al. Treatment of hyperprolactinaemia reduces total cholesterol and LDL in patients with prolactinomas. Metab Brain Dis. 2017;32:155–61.
Yedinak CG, Cetas I, Ozpinar A, McCartney S, Dogan A, Fleseriu M. Dopamine agonist therapy induces significant recovery of HPA axis function in prolactinomas independent of tumor size: a large single center experience. Endocrine. 2016;54:191–7.
Shimon I, Sosa E, Mendoza V, Greenman Y, Tirosh A, Espinosa E, et al. Giant prolactinomas larger than 60 mm in size: a cohort of massive and aggressive prolactin-secreting pituitary adenomas. Pituitary. 2016;19:429–36.
Espinosa E, Sosa E, Mendoza V, Ramırez C, Melgar V, Mercado MS. Giant prolactinomas: are they really different from ordinary macroprolactinomas? Endocrine. 2016;52:652–9.
Mallea-Gil MS, Manavela M, Alfieri A, Ballarino MC, Chervin A, Danilowicz K, et al. Prolactinomas: evolution after menopause. Arch Endocrinol Metab. 2016;60:42–6.
Dogansen SC, Selcukbiricik OS, Tanrikulu S, Yarman S. Withdrawal of dopamine agonist therapy in prolactinomas: In which patients and when? Pituitary. 2016;19:303–10.
Auriemma RS, Galdiero M, Vitale P, Granieri L, Calzo FL, Salzano C, et al. Effect of chronic cabergoline treatment and testosterone replacement on metabolism in male patients with prolactinomas. Neuroendocrinology. 2015;191:66–81.
Ji MJ, Kim JH, Lee JH, Lee JH, Kim YH, Paek SH, et al. Best candidates for dopamine agonist withdrawal in patients with prolactinomas. Pituitary. 2017;20:578–84.
Paepegaey A, Salenave S, Kamenicky P, Maione L, Brailly-Tabard S, Young J, et al. Cabergoline tapering is almost always successful in patients with macroprolactinomas. J Endocrine Soc. 2017;1:221–30.
Akinduro OO, Lu VM, Izzo A, Biase GD, Vilanilam G, Gompel JJV, et al. Radiographic and hormonal regression in prolactinomas: An analysis of treatment failure. World Neurosurg. 2019;249:e1–9.
Santharam S, Fountas A, Tampourlou M, Arlt W, Ayuk J, Gittoes N, et al. Impact of menopause on outcomes in prolactinomas after dopamine agonist treatment withdrawal. Clin Endocrinol (Oxf). 2018;89:346–53.
Celik E, Ozkaya HM, Poyraz BC, Saglam T, Kadioglu P. Impulse control disorders in patients with prolactinoma receiving dopamine agonist therapy: a prospective study with 1 year follow-up. Endocrine. 2018;62:692–700.
Araújo C, Marques O, Almeida R, Santos MJ. Macroprolactinomas: longitudinal assessment of biochemical and imaging therapeutic responses. Endocrine. 2018;62:470–6.
Dogansen SC, Cikrikcili U, Oruk G, Kutbay NO, Tanrikulu S, Hekimsoy Z, et al. Dopamine agonist-induced impulse control disorders in patients with prolactinoma: A cross-sectional multicenter study. J Clin Endocrinol Metab. 2019;104:2527–34.
Shimon I, Hirsch D, Tsvetov G, Robenshtok E, Akirov A, Fraenkel M, et al. Hyperprolactinemia diagnosis in elderly men: a cohort of 28 patients over 65 years. Endocrine. 2019;65:656–61.
Marić A, Kruljac I, Čerina V, Pećina HI, Šulentić P, Vrkljan M. Endocrinological outcomes of pure endoscopic transsphenoidal surgery: A croatian referral pituitary center experience. Croat Med J. 2012;53:224–33.
Kristof RA, Schramm J, Redel L, Neuloh G, Wichers M, Klingmu¨ller D. Endocrinological outcome following first time transsphenoidal surgery for GH-, ACTH-, and PRL-secreting pituitary adenomas. Acta Neurochir (Wien). 2002;144:555–61.
Yan Z, Wang Y, Shou X, Su J, Lang L. Effect of transsphenoidal surgery and standard care on fertility related indicators of patients with prolactinomas during child-bearing period. Int J Clin Exp Med. 2015;8:21557–64.
Smith TR, Hulou MM, Huang KT, Gokoglu A, Cote DJ, Woodmansee WW, et al. Current indications for the surgical treatment of prolactinomas. J Clin Neurosci. 2015;22:1785–91.
Yoo F, Chan C, Kuan E, Bergsneider M, Wang MB. Comparison of male and female prolactinoma patients requiring surgical intervention. J Neurol Surg. 2018;79:394–400.
Cho D, Liau W. Comparison of endonasal endoscopic surgery and sublabial microsurgery for prolactinomas. Surg Neurol. 2002;58:371–6.
Liu W, Zahr RS, McCartney S, Cetas JS, Dogan A, Fleseriu M. Clinical outcomes in male patients with lactotroph adenomas who required pituitary surgery: a retrospective single center study. Pituitary. 2018;21:454–62.
Zhao Y, Jin D, Lian W, Xing B, Feng M, Liu X, et al. Clinical characteristics and surgical outcome of prolactinoma in patients under 14 years old. Medicine (Baltimore). 2019;98:e14380.
Santoro A, Minniti G, Ruggeri A, Esposito V, Jaffrain-Rea M-L, Delfini R. Biochemical remission and recurrence rate of secreting pituitary adenomas after transsphenoidal adenomectomy: long-term endocrinologic follow-up results. Surg Neurol. 2007;68:513–8.
Bevan JS, Adams CBT, Burke CW, Morton KE, Molyneux AJ, Moore RA, et al. Factors in the outcome of transsphenoidal surgery for prolactinoma and non-functioning pituitary tumour, including pre-operative bromocriptine therapy. Clin Endocrinol (Oxf). 1987;26:541–56.
Nelson PB, Goodman M, Maroon JC, Martinez AJ, Moossy J, Robinson AG. Factors in predicting outcome from operation in patients with prolactin-secreting pituitary adenomas. Neurosurgery. 1983;13:634–41.
Micko A, Vila G, Höftberger R, Knosp E, Wolfsberger S. Endoscopic transsphenoidal surgery of microprolactinomas: A reappraisal of cure rate based on radiological criteria. Neurosurgery. 2018;0:1–8.
Jho H. Endoscopic transsphenoidal surgery. J Neurooncol. 2001;54:187–95.
Gondim JA, Schops M, de Almeida JPC, Albuquerque LAF, Gomes E, Tn F, et al. Endoscopic endonasal transsphenoidal surgery: surgical results of 228 pituitary adenomas treated in a pituitary center. Pituitary. 2010;13:68–77.
Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44–51.
Wang F, Zhou T, Wei S, Zhang J, Hou Y, Sun G. Endoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomas. Surg Endosc. 2015;29:1270–80.
Hofstetter CP, Shin BJ, Mubita L, Huang C, Anand VK, Boockvar JA, et al. Endoscopic endonasal transsphenoidal surgery for functional pituitary adenomas. Neurosurg Focus. 2011;30:E10–E.
Yano S, Kawano T, Kudo M, Makino K, Nakamura H, Kai Y, et al. Endoscopic endonasal transsphenoidal approach through the bilateral nostrils for pituitary adenomas. Neurol Med Chir (Tokyo). 2009;49:1–7.
Paluzzi A, Fernandez-Miranda JC, Stefko ST, Challinor S, Snyderman CH, Gardner PA. Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary. 2014;17:307–19.
Webster J, Page MD, Bevan JS, Richards SH, Douglas-Jonest AG, Scanlon MF. Low recurrence rate after partial hypophysectomy for prolactinoma: the predictive value of dynamic prolactin function tests. Clin Eadocrinol. 1992;36:35–44.
Rodman EF, Molitch ME, Post KD, Biller BJ, Reichlin S. Long-term follow-up of transsphenoidal selective adenomectomy for prolactinoma. J Am Med Assoc. 1984;252:921–4.
Ciccarelli E, Ghigo E, Miola C, Gandini G, Muller EE, Camanni F. Long-term follow-up of ‘cured’ prolactinoma patients after successful adenomectomy. Clin Endocrinol (Oxf). 1990;32:583–92.
Schlechte JA, Sherman BM, Chapler FK, Van Gilder J. Long term follow-up of women with surgically treated prolactin- secreting pituitary tumors. J Clin Endocrinol Metab. 1986;62:1296–301.
Nakagawa H, Iwatsuki K, Yamada M, Hagiwara Y, Moriuchi S, Kadota T. Latent prolactinoma on MRI-selective venous sampling and trans-sphenoidal microsurgical treatment. Neurol Res. 2001;23:691–6.
Parl FF, Cruz VE, Cobb CA, Bradley CA, Aleshire SL. Late recurrence of surgically removed prolactinomas. Cancer. 1986;57:2422–6.
Sata A, Hizuka N, Kawamata T, Hori T, Takano K. Hyponatremia after transsphenoidal surgery for hypothalamo-pituitary tumors. Neuroendocrinology. 2006;83:117–22.
Arafah BUM, Brodkey JS, Pearson OH. Gradual recovery of lactotroph responsiveness following surgical removal of prolactinomas. Metabolism. 1986;35:905–12.
Hirohata T, Uozumi T, Mukada K, Arita K, Kurisu K, Yano T, et al. Influence of pregnancy on the serum prolactin level following prolactinoma surgery. Acta Endocrinol (Copenh). 1991;125:259–67.
Massoud F, Serri O, Hardy J, Somma M, Beauregard H. Transsphenoidal adenomectomy for microprolactinomas 10 to 20 years of follow-up. Surg Neurol. 1996;45:341–6.
Fraioli MF, Umana G, Pagano A, Fraioli B, Lunardi P. Prolactin secreting pituitary microadenoma: Results of transsphenoidal surgery after medical therapy with dopamine agonist. J Craniofac Surg. 2017;28:992–4.
Raverot G, Wierinckx A, Dantony E, Auger C, Chapas G, Villeneuve L, et al. Prognostic factors in prolactin pituitary tumors: Clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocrinol Metab. 2010;95:1708–16.
Fahlbusch R, Buchfelder M. Present status of neurosurgery in the treatment of prolactinomas. Neurosurg Rev. 1985;8:195–205.
Sughrue ME, Chang EF, Tyrell JB, Kunwar S, Wilson CB Jr, LSB. Pre-operative dopamine agonist therapy improves post-operative tumor control following prolactinoma resection. Pituitary. 2009;12:158–64.
Babey M, Sahli R, Vajtai I, Andres RH, Seiler RW. Pituitary surgery for small prolactinomas as an alternative to treatment with dopamine agonists. Pituitary. 2011;14:222–30.
Pelkonen R, Grahne B, Hirvonen E, Karonen S-L, Salmi J, Tikkanen M, et al. Pituitary function in prolactinoma. effect of surgery and postoperative bromocriptine therapy. Clin Eadocrinol. 1980;14:335–48.
Primeau V, Raftopoulos C, Maiter D. Outcomes of transsphenoidal surgery in prolactinomas: improvement of hormonal control in dopamine agonist-resistant patients. Eur J Endocrinol. 2012;166:779–86.
Kreutzer J, Buslei R, Wallaschofski H, Hofmann B, Nimsky C, Fahlbusch R, et al. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. 2008;158:11–8.
Kawamata T, Iseki H, Ishizaki R, Hori T. Minimally invasive endoscope-assisted endonasal trans-sphenoidal microsurgery for pituitary tumors: Experience with 215 cases comparing with sublabial trans-sphenoidal approach. Neurol Res. 2002;24:259–65.
Wolfsberger S, Czech T, Vierhapper H, Benavente R, Knosp E. Microprolactinomas in males treated by transsphenoidal surgery. Acta Neurochir (Wien). 2003;145:935–41.
Mamelak AN, Carmichael J, Bonert VH, Cooper O, Melmed S. Single-surgeon fully endoscopic endonasal transsphenoidal surgery: outcomes in three-hundred consecutive cases. Pituitary. 2013;16:393–401.
Han Y, Chen D, Zhang C, Pan M, Yang X-P, Wu Y-G. Retrospective analysis of 52 patients with prolactinomas following endoscopic endonasal transsphenoidal surgery. Medicine (Baltimore). 2018;97:e13198.
Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005;56:1222–33.
Koizumi K, Aono T, Koike K, Kurachi K. Restoration of LH pulsatility in patients with prolactinomas after trans-sphenoidal surgery. Acta Endocrinol (Copenh). 1984;107:433–8.
Serri O, Rasio E, Beauregard H, Hardy J, Somma M. Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med. 1983;309:280–3.
Akin S, Isikay I, Soylemezoglu F, Yucel T, Gurlek A, Berker M. Reasons and results of endoscopic surgery for prolactinomas: 142 surgical cases. Acta Neurochir (Wien). 2016;158:933–42.
Dehdashti AR, Ganna A, Karabatsou K, Gentili F. Pure endoscopic endonasal approach for pituitary adenomas early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery. 2007;62:1006–17.
Woosley RE, King JS, Talbert L. Prolactin-secreting pituitary adenomas: neurosurgical management of 37 patients. Fertil Steril. 1982;37:54.
Maira G, Anile C, De Marinis L. Prolactin-secreting adenomas: surgical results and long-term follow-up. Neurosurgery. 1989;24:736–43.
Liu Y, Yao Y, Xing B, Lian W, Deng K, Feng M, et al. Prolactinomas in children under 14. Clinical presentation and long-term follow-up. Childs Nerv Syst. 2015;31:909–16.
Faria JMA, Tindall GT. Transsphenoidal microsurgery for prolactin-secreting pituitary adenomas. J Neurosurg. 1982;56:33.
Esposito V, Santoro A, Minniti G, Salvati M, Innocenzi G, Lanzetta G, et al. Transsphenoidal adenomectomy for GH-, PRL- and ACTH-secreting pituitary tumours: outcome analysis in a series of 125 patients. Neurol Sci. 2004;25:251–6.
Soule SG, Farhi J, Conway GS, Jacobs HS, Powell M. The outcome of hypophysectomy for prolactinomas in the era of dopamine agonist therapy. Clin Endocrinol (Oxf). 1996;44:711–6.
Frank G, Pasquini E, Farneti G, Mazzatenta D, Sciarretta V, Grasso V, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83:240–8.
Losa M, Mortini P, Barzaghi R, Gioia L, Giovanelli M. Surgical treatment of prolactin-secreting pituitary adenomas: Early results and long-term outcome. J Clin Endocrinol Metab. 2002;87:3180–6.
Charpentier G, de Plunkett T, Jedynak P, Peillon F, Le Gentil P, Racadot J, et al. Surgical treatment of prolactinomas short- and long-term results, prognostic factors. Horm Res Paediatr. 1985;22:222–7.
Song Y, Chen M, Lian W, Xing B, Yao Y, Feng M, et al. Surgical treatment for male prolactinoma A retrospective study of 184 cases. Medicine (Baltimore). 2017;96:e5833.
Donegan D, Atkinson JLD, Jentoft M, Natt N, Nippoldt TB, Erickson B, et al. Surgical outcomes of prolactinomas in recent era: Results of a heterogenous group. Endocr Pract. 2017;23:37–45.
Hamilton DK, Vance ML, Boulos PT, Laws ER. Surgical outcomes in hyporesponsive prolactinomas: Analysis of patients with resistance or intolerance to dopamine agonists. Pituitary. 2005;8:53–60.
Qu X, Wang M, Wang G, Han T, Mou C, Han L, et al. Surgical outcomes and prognostic factors of transsphenoidal surgery for prolactinoma in men: a single-center experience with 87 consecutive cases. Eur J Endocrinol. 2011;164:499–504.
Saitoh Y, Mori S, Arita N, Nagatani M, Hayakawa T, Koizumi K, et al. Treatment of prolactinoma based on the results of transsphenoidal operations. Surg Neurol. 1986;26:338–44.
Thomson JA, Teasdale GM, Gordon D, Mccruden DC, Davies DL. Treatment of presumed prolactinoma by transsphenoidal operation: early and late results. Br Med J. 1985;291:1550–3.
Tamasauskas A, Sinkunas K, Bunevicius A, Radziunas A, Skiriute D, Deltuva VP. Transsphenoidal surgery for microprolactinomas in women: results and prognosis. Acta Neurochir (Wien). 2012;154:1889–93.
Turner HE, Adams CB, Wass JA. Trans-sphenoidal surgery for microprolactinoma: an acceptable alternative to dopamine agonists? Eur J Endocrinol. 1999;140:43–7.
Ikeda H, Watanabe K, Tominaga T, Yoshimoto T. Transsphenoidal microsurgical results of female patients with prolactinomas. Clin Neurol Neurosurg. 2013;115:1621–5.
Yi N, Ji L, Zhang Q, Zhang S, Liu X, Shou X, et al. Long-term follow-up of female prolactinoma patients at childbearing age after transsphenoidal surgery. Endocrine. 2018.
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–88.
Maiter D, Primeau V. 2012 update in the treatment of prolactinomas. Annales d'Endocrinologie. 2012;73:90–8.
Cappabianca P, Cavallo LM, Solari D. Transsphenoidal surgery: A journey of 50 years. World Neurosurg. 2013;79:253–4.
Maiter D, Delgrange E. Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol. 2014;170:R213–27.
Duan L, Yan H, Huang M, Zhang Y, Gu F. An economic analysis of bromocriptine versus trans-sphenoidal surgery for the treatment of prolactinoma. J Craniofac Surg. 2017;28:1046–51.
Jingran Z, Qi Y, Yuhui Z. Cost-effectiveness analysis of two therapeutic methods for prolactinoma. Chin J Obstet Gynecol. 2008;43:257–61.
Zygourakis CC, Imber BS, Chen R, Han SJ, Blevins L, Molinaro A, et al. Cost-effectiveness analysis of surgical versus medical treatment of prolactinomas. J Neurol Surg. 2017;78:125–31.
Acknowledgements
Special thanks to Peiqing Cai and Wenjing Gou for their support in this research.
Funding
No grant supported this study.
Availability of data and materials
Not applicable.
Authors’ contributions
Chiyuan Ma conceived and designed the investigation. Xiangming Cai analyzed the data and drafted the manuscript. Junhao Zhu, Jin Yang, Chao Tang, and Zixiang Cong conducted statistical analyses. The authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Supplementary Figure 1
. A. Summary of Risk of bias assessment for randomized controlled trials using ROB.2 tool. B. Summary of Risk of Bias assessment for non-randomized controlled trials using ROBINS-I tool.
Additional file 2: Supplementary Figure 2.
Forest plots for subgroup analysis of biochemical cure rates in surgery-treated patients subgrouped by patients type (A), publication years (B), surgery types (C); and in DAs-treated patients subgrouped by patients type (D), publication years (E), DAs types (F).
Additional file 3: Supplementary Figure 3.
Funnel plots for biochemical cure rate of patients treated with surgery (A) and DAs (B).
Additional file 4: Supplementary Figure 4.
Forest plots for subgroup analysis of recurrence rates in surgery-treated patients subgrouped by patients type (A), publication years (B), surgery types (C); and in DAs-treated patients subgrouped by patients type (D), publication years (E), DAs types (F).
Additional file 5: Supplementary Figure 5.
Forest plots for prolactin level of patients applying surgery (A) and DAs (B).
Additional file 6: Supplementary Figure 6.
Forest plots for improvement rates for vision impairment (A), headache (B), menstrual disturbance (C), galactorrhoea (D) and incidence rates of ACTH insufficiency (E), TSH deficiency (F), hypopituitarism (G), diabetes insipidus (H) of patients applying surgery.
Additional file 7: Supplementary Figure 7.
Forest plots for improvement rates for vision impairment (A), headache (B), menstrual disturbance (C), galactorrhoea (D) and incidence rates of ACTH insufficiency (E), TSH deficiency (F), hypopituitarism (G) of patients applying DAs.
Additional file 8: Supplementary Figure 8.
Forest plots for subgroup analysis of biochemical cure rates in surgery-treated patients subgrouped by DAs treatment history.
Additional file 9: Supplementary Table 1.
Basic characteristics of the included studies with surgery treatment.
Additional file 10: Supplementary Table 2.
Basic characteristics of the included studies with DAs treatment.
Additional file 11: Supplementary Table 3.
Summary table of risk of bias for RCT.
Additional file 12: Supplementary Table 4.
Summary table of risk of bias for non-RCT.
Additional file 13: Supplementary Table 5.
Summary table of risk of bias for case-series study.
Additional file 14: Supplementary file 1.
Literature research strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cai, X., Zhu, J., Yang, J. et al. Are dopamine agonists still the first-choice treatment for prolactinoma in the era of endoscopy? A systematic review and meta-analysis. Chin Neurosurg Jl 8, 9 (2022). https://doi.org/10.1186/s41016-022-00277-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41016-022-00277-1