Abstract
Background
The fluid challenge is considered the gold standard for diagnosis of fluid responsiveness. The objective of this study was to describe the fluid challenge techniques reported in fluid responsiveness studies and to assess the difference in the proportion of ‘responders,’ (PR) depending on the type of fluid, volume, duration of infusion and timing of assessment.
Methods
Searches of MEDLINE and Embase were performed for studies using the fluid challenge as a test of cardiac preload with a description of the technique, a reported definition of fluid responsiveness and PR. The primary outcome was the mean PR, depending on volume of fluid, type of fluids, rate of infusion and time of assessment.
Results
A total of 85 studies (3601 patients) were included in the analysis. The PR were 54.4% (95% CI 46.9–62.7) where <500 ml was administered, 57.2% (95% CI 52.9–61.0) where 500 ml was administered and 60.5% (95% CI 35.9–79.2) where >500 ml was administered (p = 0.71). The PR was not affected by type of fluid. The PR was similar among patients administered a fluid challenge for <15 minutes (59.2%, 95% CI 54.2–64.1) and for 15–30 minutes (57.7%, 95% CI 52.4–62.4, p = 1). Where the infusion time was ≥30 minutes, there was a lower PR of 49.9% (95% CI 45.6–54, p = 0.04). Response was assessed at the end of fluid challenge, between 1 and 10 minutes, and >10 minutes after the fluid challenge. The proportions of responders were 53.9%, 57.7% and 52.3%, respectively (p = 0.47).
Conclusions
The PR decreases with a long infusion time. A standard technique for fluid challenge is desirable.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Intravenous fluid is one of the most commonly administered therapies for critically ill patients and is the cornerstone of haemodynamic management of patients in intensive care units (ICUs) [1]. The rationale for volume expansion is to increase the cardiac output (CO) and oxygen delivery to ultimately improve tissue oxygenation. The gold standard for assessing fluid responsiveness to guide fluid administration in critically ill patients is to perform a fluid challenge. This involves the infusion of a specific amount of intravenous fluid to assess ventricular preload reserve and subsequent systemic haemodynamic effects [2]. The volume of fluid infused must be sufficient to increase right ventricular diastolic volume and subsequently stroke volume (SV) as described by the Frank-Starling law [3]. Fluid responsiveness is conventionally defined as an increase of at least 10% to 15% in SV in response to a fluid challenge, which is a reflection of the limits of precision of the technology used [4, 5]. Patients who reach this threshold are considered ‘fluid responders’. Clinical studies have demonstrated that approximately 50% of critically ill patients who are deemed to have inadequate CO are fluid responders [6]. However, fluid responsiveness is neither a binary nor a static condition, because it depends on dynamic interaction between intravascular volume, vascular tone and ventricular function. Furthermore, fluid responsiveness may also depend on the particularities of the fluid challenge, including the type and volume of fluid as well as the administration rate.
Administration of a fluid challenge is not a standardised technique, with varying volumes, infusion rates, fluid types and durations of response. The use of different methods to estimate SV is a further confounder. Whilst different clinical conditions may require different fluid challenge techniques, there is heterogeneity in practice for the same clinical condition [6].
We hypothesise that the technique of fluid challenge affects fluid responsiveness. This may result in different clinical decisions. Either inadequate or excessive fluid administration has adverse clinical consequences, and a better understanding fluid administration is likely to improve patient management and outcome. The objective of this study was to describe the different fluid challenge techniques used in clinical trials by assessing fluid responsiveness and how the proportion of patients deemed ‘fluid-responsive’ varies according to the technique used.
Methods
Studies
This study was conducted following a pre-defined protocol (Additional file 1: Appendix 1). No ethical approval or patient consent was necessary for the present study. We included studies meeting the following inclusion criteria: use of a fluid challenge as a test of cardiac preload or as part of a clinical algorithm, studies performed in ICUs or operating theatres with adult patients, studies including a full description of the fluid challenge technique (volume, infusion rate, type of fluid used and timing of assessment of the haemodynamic response), studies which included a clear definition of fluid responsiveness, and studies where the numbers of responders and non-responders to the fluid challenge were stated. Only studies published as full-text articles, published in English and in an indexed journal were included. Reviews, case reports and studies published in abstract form were excluded. We excluded studies involving pregnant women and children, studies where more than one fluid challenge was performed in the same patient, studies involving passive leg raising without use of a fluid challenge technique, studies where more than one fluid type was used whilst reporting a single result, studies using a continuous infusion of fluid, and studies where the fluid responsiveness was assessed only after a period of 60 minutes or more following completion of fluid challenge. Studies reporting more than one type of fluid challenge with a full description of results for each type of fluid challenge used were included for analysis as two separate studies. Studies reporting more than one type of fluid challenge (i.e., colloids and crystalloids) without a full description of results for each type of fluid challenge were excluded from the relevant part of the analysis (i.e., type of fluid).
Search strategy and data extraction
Three of the authors (LT, DA and DB) conducted a computerised search of the MEDLINE and Embase databases in February 2016. The terms included for the research were used in the following Boolean operators: ‘fluid challenge’ OR ‘fluid bolus’ OR ‘fluid therapy’ OR ‘fluid responsiveness’ OR ‘fluid resuscitation’ AND ‘intensive care’ OR ‘critical care’ OR ‘operative theatre’ OR ‘anaesthesia’ AND ‘stroke volume’ OR ‘cardiac output’ OR ‘cardiac index’ OR ‘stroke volume variation’ OR ‘pulse pressure variation’ OR ‘stroke pressure variation’. The search was filtered by language, the age of participants (adults) and the availability of full-text articles using the native filter function of each database used.
Titles and abstracts of the trials identified in the search were independently reviewed and pooled for further screening. The full text of each trial identified was analysed, and each reviewer compiled a list of studies that met the inclusion criteria. Each review author’s list was compared, and any disagreement was resolved through discussions until a consensus was reached among all review authors.
The following data were extracted from each study: volume of fluid used in the fluid challenge, duration of the infusion, type of fluid used, definition of fluid responsiveness, methodology used for the fluid responsiveness assessment, characteristics of the patients enrolled in the study, clinical environment in which the study was performed, number of patients included in the study, and percentage of ‘fluid responders’. Data were extracted independently by three authors (LT, DA and DB) and verified by another author (HDA).
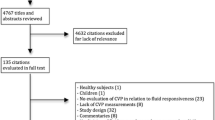
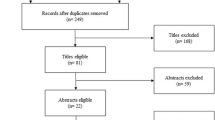
The identification, screening and inclusion of studies in this review are summarised in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram in Fig. 1. A PRISMA checklist is also reported in Additional file 1.
Statistical analysis
Data were examined graphically and statistically (Shapiro-Wilk test) to understand the distribution and nature of each variable. Data are presented as mean and 95% CI when normally distributed or as median and IQR for non-parametric data. Not all the studies reported the data required for the analysis of all the outcomes. Whenever any data were missing, only those studies with the data reported for the relevant analysis were included. Not imputation technique was applied.
The primary outcome of the study was the difference in means of proportion of fluid responders (PR). The included studies were grouped into three categories on the basis of volume used for the fluid challenge: <500 ml, 500 ml and >500 ml. Studies were grouped into three categories for the duration of the fluid infusion: <15 minutes, between 15 and 30 minutes and ≥30 minutes. Cut-off values for the duration and volume of fluids infused were defined following review of the literature. The types of fluid used were grouped into two categories: colloids and crystalloids.
Two-way independent analysis of variance (ANOVA) was conducted to compare means and variances between groups using as second variable (the setting of the study: ICU vs theatre), given the potential different pathophysiology of these two groups and the potential impact on the PR. Bootstrapping was conducted using 1000 samples and bias-corrected and accelerated. When assumptions for two-way independent ANOVA were not met, one-way independent ANOVA results are reported. Post hoc test results are reported with Bonferroni correction for multiple comparisons. Statistical significance was considered at a p value <0.05. Statistical analysis was performed using IBM SPSS Statistics version 24 software (IBM, Armonk, NY, USA).
Results
A total of 363 titles were identified through PubMed, and 163 were identified through Embase. After removal of duplications, 404 titles were collected for the analysis (Fig. 1). Screening by title and abstract excluded 233 studies, and 171 studies were selected for full-text assessment. Three studies were identified by snowballing. Eighty-five studies were selected for the final analysis. Two different sets of data were extracted from three studies because two different fluid challenge techniques were reported with the respective proportions of responders and non-responders. In total, 88 sets of data extracted from 85 studies with an aggregated 3601 patients were analysed (Table 1).
The definition of positive response to a fluid challenge varies substantially across studies (Additional file 1: Figure S1). Physiological parameters used to assess fluid response include cardiac index (47.5%), CO (17.1%), SV (11.0%) and stroke volume index (24.3%). The increment from baseline measurements in physiological parameters deemed to have a positive response to a fluid challenge was either 10% (25.5% of studies) or 15% (74.5% of studies). The most frequent definition of a positive response to a fluid challenge was an increase in cardiac index of at least 15% from baseline (n = 33 [40.2%]). CO was estimated using several different technologies (Additional file 1: Figure S2), with pulse index continuous CO (PiCCO; PULSION Medical Systems, Feldkirchen, Germany) used most frequently (31.7% of studies), followed by the pulmonary artery catheter (PAC; 22% of studies) (Table 1). There was a higher percentage of responders in studies performed in the operating room (63.4%, 95% CI 58.3–68.4) than in the ICU (51.5%, 95% CI 48.2–54.8, p < 0.001).
Volume of fluid challenge
The volumes of fluid administered for the fluid challenge varied from <500 ml (n = 8 [12.7%]) to 500 ml (n = 50 [79.4%]) and >500 ml (n = 5 [7.9%]). Twenty-four studies were excluded from this analysis because the volume was described as milligrams per kilogram and the participants’ body weight was not reported. The estimated mean PR values were 54.4% (95% CI 46.9–62.7) among patients receiving <500 ml, 57.2% (95% CI 52.9–61.0) among patients receiving 500 ml and 60.5% (95% CI 35.9–79.2) among patients receiving >500 ml. There was no difference in the PR values between groups of patients receiving different volumes of fluid challenges [F(2,57) = 0.35, p =0.71] (Additional file 1: Figure S3). The PR observed in studies where the fluid was prescribed as a fixed volume (n = 63 [72.4%]) and where fluid volume was adjusted for body weight (n = 24 [27.6%]) was similar [F (1,83) = 0.02, p = 0.88].
Type of fluid
Twenty-six (35%) studies used crystalloids, and 50 (65%) used colloids. Nine studies were excluded from the analysis because they used both types of fluids. Among patients receiving crystalloids, 53.5% (95% CI 45.4–58.5) were responders, as compared with 59.0% (95% CI 55.5–62.9) in the group receiving colloids (Additional file 1: Figure S4). The type of fluid used did not affect the proportion of patients responding to a fluid challenge [F(1,76) = 2.19, p = 0.14].
Duration of infusion
The time of infusion was <15 minutes in 24 studies (27.3%), between 15 and 29 minutes in 26 studies (29.5%), and ≥30 minutes in 29 studies (33%). Nine studies (10.2%) did not report duration of infusion. Where the fluid challenge was administered for <15 minutes, between 15 and 29 minutes, and >30 minutes, the proportions of patients deemed to be fluid responders were 59.2% (95% CI 54.2–64.1), 57.7% (95% CI 52.4–62.4), and 49.9% (95% CI 45.6–54) respectively. The duration of the fluid infusion affects the proportion of fluid responders [F(2,73) = 3.63, p = 0.03] (Fig. 2). The PR to a fluid challenge given in ≥30 minutes was lower than the PR when the fluid challenge was given in <15 minutes (p = 0.045). The proportion of patients responding to a fluid challenge that was administered in <15 minutes and between 15 and 30 minutes was similar (p = 1.0).
Timing of assessment
The assessment of response to a fluid challenge was at the point of administration (n = 50 [58.1%]), between 1 and 10 minutes (n = 31 [36.8%]), or >10 minutes (n = 5 [5.8%]) after completion of the fluid challenge. Where fluid responsiveness was assessed at the point of administration, between 1 and 10 minutes, and >10 minutes after completion of the fluid challenge, 53.9% (95% CI 49.8–57.7), 57.7% (95% CI 52.9–62.7), and 52.3% (95% CI 32–90.5) of patients had a positive response, respectively. The time of assessment of fluid response did not affect the PR [F(2,80) = 0.76, p = 0.47] (Fig. 3).
Discussion
We demonstrate that the duration of the fluid infusion in a fluid challenge has a significant influence on fluid responsiveness. This confirms our hypothesis that the proportion of patients deemed to respond to a fluid challenge is influenced by the characteristics of a fluid challenge technique, in addition to intravascular filling, vascular tone or ventricular contractility. Other aspects of the fluid challenge, including the volume, type of fluid or assessment time, do not affect the proportion of patients who are fluid responders. Currently, no consensus exists on how to perform an effective fluid challenge. This study highlights the need for a standardised technique for research and clinical purposes.
Fluid challenge is one of the commonest interventions in critical care medicine. A recent international observational study [6] including 2279 patients from 311 centres highlighted the variability in this intervention. In contrast to our results, crystalloids were more frequently used (74.0%), with balanced solutions used in most of cases (53.3%). The study was undertaken following the publication of large, randomised clinical trials advocating the use of crystalloids over colloids [7,8,9,10]. Up to two to three times as much crystalloid as colloid may be required to maintain intravascular volume, owing to differences in intravascular half-life [11]. Fluid challenges consisting of colloids compared with crystalloids are associated with a more linear increase in cardiac filling and SV compared with crystalloids [12].
However, the theoretical benefits of colloids over crystalloids in critically ill patients with altered endothelial permeability have not been borne out in clinical trials. Starch-based solutions are associated with increased rates of acute kidney injury and coagulopathy compared with crystalloid solutions [7, 8, 13]. Human albumin solution is associated with a poorer prognosis in patients with traumatic brain injury [14] and is not associated with any survival benefit compared with colloids in patients with sepsis [15]. We did not find any difference in PR by the type of fluid used for a fluid challenge. If the time of assessment of fluid responsiveness is immediately after fluid infusion or in the first minutes, it is unlikely that the type of fluid would make any difference, because in both cases (colloids/crystalloids) it is likely that a big proportion of the volume infused will remain in the intravascular compartment. If the assessment of fluid responsiveness were performed later, it would be possible to observe some differences because theoretically colloids remain longer in the intravascular space than crystalloids do. This would require further investigation.
Consistent with a recent large observational study [6], the most common volume of fluid used for a fluid challenge was 500 ml. However, there was significant variability in the volume of fluid used. The total volume of fluid administered to determine fluid responsiveness varies widely, from 4 to 20 ml/kg or 100 to 1000 ml. Whilst fluid challenge with larger volumes may have serious clinical consequences, such as pulmonary oedema, very small volumes may not represent a cardiovascular challenge. The clinical challenge lies in determining the optimal volume of fluid required to optimise cardiac performance and tissue perfusion. The effect of the volume of fluid challenge was recently investigated by our group [16]. Eighty patients were administered four different volumes as fluid challenges (1, 2, 3 and 4 ml/kg of crystalloids) over 5 minutes. Pmsf-arm, a surrogate of the mean systemic filling pressure (Pmsf), was measured. Pmsf itself is a measure of effective intravascular filling independent of cardiac function [17]. This technique has been shown to be precise for a change of 14% from baseline [18]. The minimal volume required to achieve an increment of 14% was 4 ml/kg. Importantly, the dose of fluids used affects the change in CO and consequently the proportion of patients considered to be responsive to a fluid challenge. Differences in the volume of fluid required to achieve a positive fluid response between this study and other studies in this meta-analysis may be explained by the heterogeneity in the methods used for estimating CO, thresholds defining a positive response, patient case mix and illness severity.
The optimal rate of fluid infusion is unknown. The researchers in the Fluid Challenges in Intensive Care (FENICE) study [6] reported a median infusion time of 24 minutes to administer a fluid challenge. Our results suggest that the duration of the fluid infusion has a significant effect on observed fluid responders. An infusion time <30 minutes is more effective in detecting fluid responders than infusion times >30 minutes. These results are consistent with our understanding of cardiovascular physiology, where a rapid intravenous fluid bolus will rapidly increase venous return to increase right ventricular end-diastolic volume. A slower rate of infusion, however, would result in a lower increase of venous return and result in a lower rise in SV, thus becoming less effective. Prospective clinical studies are warranted before these findings can be incorporated into routine clinical practice.
Pooled data in this meta-analysis indicate that the timing of assessment of a fluid challenge does not have a significant impact on detecting a positive response. This is in contrast to previous work by our group in which the haemodynamic effect of a 250-ml crystalloid fluid challenge was almost completely dissipated after 10 minutes from the end of the fluid challenge [19]. In this meta-analysis, many studies used PAC as a method to estimate CO, which cannot accurately detect immediate changes in SV. This makes it more challenging to study the immediate physiological effect of the fluid challenge on SV. A more sustained response would intuitively be clinically favourable. However, this is likely to be influenced by the patient’s underlying pathophysiology in addition to the fluid challenge technique itself. In this study, it is possible to comment only on the physiological effect of the fluid challenge, because the clinical effect is beyond the scope of this review. Another possible explanation for the discrepancy in results is the distribution of studies between categories of the assessment time: only five studies reported a time of assessment after 10 minutes, which is the time point at which we have previously observed complete dissipation of the haemodynamic effect of the fluid challenge.
As with all retrospective observational studies, the data presented must be interpreted in the context of its limitations. There is likely to be significant heterogeneity in the patient case mix, illness severity and overall management. Different permutations of the rate of fluid administered, the type and volume of fluid, method of haemodynamic assessment, threshold for definition of responsiveness, and the time of assessment of fluid challenge does not allow any strong conclusions to be made. Furthermore, we have not accounted for the different methods of haemodynamic monitoring used. However, we highlight the heterogeneity in practice of this commonly applied technique and the need for further investigation to elucidate the clinical effect of the different aspects of a fluid challenge.
Conclusions
The proportion of patients who respond to a fluid challenge is dependent on the particularities of the technique used. A rapid infusion of fluid volume increases the proportion of patients with a positive response. However, the type and volume of fluid or the time of assessment does not appear to have any effect on the detection of fluid responders. This study highlights that standardisation of the fluid challenge technique is needed for contextualisation of clinical trial data and patient management.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CO:
-
Cardiac output
- FC:
-
Fluid challenge
- ICG:
-
Impedance cardiography
- ICU:
-
Intensive care unit
- N/A:
-
Not available
- ODM:
-
Oesophageal Doppler monitoring
- PAC:
-
Pulmonary artery catheter
- Pmsf:
-
Mean systemic filling pressure
- PR:
-
Proportion of responders
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SV:
-
Stroke volume
- SVI:
-
Stroke volume index
- TOE:
-
Trans-oesophageal echocardiography
- TTE:
-
Trans-thoracic echocardiography
- USCOM:
-
Transcutaneous aortic Doppler ultrasonic cardiac output monitor
References
Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(25):2462–3.
Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care. 2011;17(3):290–5.
Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48(5):357–79.
Cecconi M, Dawson D, Grounds RM, Rhodes A. Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Intensive Care Med. 2009;35(3):498–504.
Squara P, Cecconi M, Rhodes A, Singer M, Chiche JD. Tracking changes in cardiac output: methodological considerations for the validation of monitoring devices. Intensive Care Med. 2009;35(10):1801–8.
Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med. 2015;41(9):1529–37.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34.
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–11.
Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S, Committee CM. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 2013;39(4):558–68.
Reinhart K, Perner A, Sprung CL, Jaeschke R, Schortgen F, Johan Groeneveld AB, Beale R, Hartog CS, European Society of Intensive Care M. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med. 2012;38(3):368–83.
Marx G, Cobas Meyer M, Schuerholz T, Vangerow B, Gratz KF, Hecker H, Sumpelmann R, Rueckoldt H, Leuwer M. Hydroxyethyl starch and modified fluid gelatin maintain plasma volume in a porcine model of septic shock with capillary leakage. Intensive Care Med. 2002;28(5):629–35.
Trof RJ, Sukul SP, Twisk JW, Girbes AR, Groeneveld AB. Greater cardiac response of colloid than saline fluid loading in septic and non-septic critically ill patients with clinical hypovolaemia. Intensive Care Med. 2010;36(4):697–701.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39.
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, Investigators SS. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56.
Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–21.
Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic Effect of Different Doses of Fluids for a Fluid Challenge: A Quasi-Randomized Controlled Study. Crit Care Med. 2017;45(2):e161–8.
Aya HD, Cecconi M. Can (and should) the venous tone be monitored at the bedside? Curr Opin Crit Care. 2015;21(3):240–4.
Aya HD, Rhodes A, Fletcher N, Grounds RM, Cecconi M. Transient stop-flow arm arterial-venous equilibrium pressure measurement: determination of precision of the technique. J Clin Monit Comput. 2016;30(1):55–61.
Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44(5):880–91.
Auler Jr JO, Galas F, Hajjar L, Santos L, Carvalho T, Michard F. Online monitoring of pulse pressure variation to guide fluid therapy after cardiac surgery. Anesth Analg. 2008;106(4):1201–6. table of contents.
Barbier C, Loubieres Y, Schmit C, Hayon J, Ricome JL, Jardin F, Vieillard-Baron A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740–6.
Belloni L, Pisano A, Natale A, Piccirillo MR, Piazza L, Ismeno G, De Martino G. Assessment of fluid-responsiveness parameters for off-pump coronary artery bypass surgery: a comparison among LiDCO, transesophageal echochardiography, and pulmonary artery catheter. J Cardiothorac Vasc Anesth. 2008;22(2):243–8.
Biais M, Bernard O, Ha JC, Degryse C, Sztark F. Abilities of pulse pressure variations and stroke volume variations to predict fluid responsiveness in prone position during scoliosis surgery. Br J Anaesth. 2010;104(4):407–13.
Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F. Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth. 2008;101(6):761–8.
Biais M, Cottenceau V, Stecken L, Jean M, Ottolenghi L, Roullet S, Quinart A, Sztark F. Evaluation of stroke volume variations obtained with the pressure recording analytic method. Crit Care Med. 2012;40(4):1186–91.
Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Henaine R, Lehot JJ. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108(2):513–7.
Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, Lehot JJ. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200–6.
Cannesson M, Attof Y, Rosamel P, Desebbe O, Joseph P, Metton O, Bastien O, Lehot JJ. Respiratory variations in pulse oximetry plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology. 2007;106(6):1105–11.
Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, Della Rocca G, Grounds RM, Rhodes A. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol. 2012;78(5):527–33.
Charbonneau H, Riu B, Faron M, Mari A, Kurrek MM, Ruiz J, Geeraerts T, Fourcade O, Genestal M, Silva S. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Crit Care. 2014;18(5):473.
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517–23.
de Waal EE, Rex S, Kruitwagen CL, Kalkman CJ, Buhre WF. Dynamic preload indicators fail to predict fluid responsiveness in open-chest conditions. Crit Care Med. 2009;37(2):510–5.
Desgranges FP, Desebbe O, Ghazouani A, Gilbert K, Keller G, Chiari P, Robin J, Bastien O, Lehot JJ, Cannesson M. Influence of the site of measurement on the ability of plethysmographic variability index to predict fluid responsiveness. Br J Anaesth. 2011;107(3):329–35.
Dufour N, Chemla D, Teboul JL, Monnet X, Richard C, Osman D. Changes in pulse pressure following fluid loading: a comparison between aortic root (non-invasive tonometry) and femoral artery (invasive recordings). Intensive Care Med. 2011;37(6):942–9.
Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834–7.
Fellahi JL, Fischer MO, Dalbera A, Massetti M, Gerard JL, Hanouz JL. Can endotracheal bioimpedance cardiography assess hemodynamic response to passive leg raising following cardiac surgery? Ann Intensive Care. 2012;2(1):26.
Fellahi JL, Fischer MO, Rebet O, Dalbera A, Massetti M, Gerard JL, Hanouz JL. Cerebral and somatic near-infrared spectroscopy measurements during fluid challenge in cardiac surgery patients: a descriptive pilot study. J Cardiothorac Vasc Anesth. 2013;27(2):266–72.
Fellahi JL, Fischer MO, Rebet O, Massetti M, Gerard JL, Hanouz JL. A comparison of endotracheal bioimpedance cardiography and transpulmonary thermodilution in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2012;26(2):217–22.
Fischer MO, Pelissier A, Bohadana D, Gerard JL, Hanouz JL, Fellahi JL. Prediction of responsiveness to an intravenous fluid challenge in patients after cardiac surgery with cardiopulmonary bypass: a comparison between arterial pulse pressure variation and digital plethysmographic variability index. J Cardiothorac Vasc Anesth. 2013;27(6):1087–93.
Fischer MO, Pellissier A, Saplacan V, Gerard JL, Hanouz JL, Fellahi JL. Cephalic versus digital plethysmographic variability index measurement: a comparative pilot study in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2014;28(6):1510–5.
Fischer MO, Coucoravas J, Truong J, Zhu L, Gerard JL, Hanouz JL, Fellahi JL. Assessment of changes in cardiac index and fluid responsiveness: a comparison of Nexfin and transpulmonary thermodilution. Acta Anaesthesiol Scand. 2013;57(6):704–12.
Geerts BF, Maas J, de Wilde RB, Aarts LP, Jansen JR. Arm occlusion pressure is a useful predictor of an increase in cardiac output after fluid loading following cardiac surgery. Eur J Anaesthesiol. 2011;28(11):802–6.
Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
Guerin L, Teboul JL, Persichini R, Dres M, Richard C, Monnet X. Effects of passive leg raising and volume expansion on mean systemic pressure and venous return in shock in humans. Crit Care. 2015;19:411.
Guinot PG, de Broca B, Abou Arab O, Diouf M, Badoux L, Bernard E, Lorne E, Dupont H. Ability of stroke volume variation measured by oesophageal Doppler monitoring to predict fluid responsiveness during surgery. Br J Anaesth. 2013;110(1):28–33.
Guinot PG, Bernard E, Defrancq F, Petiot S, Majoub Y, Dupont H, Lorne E. Mini-fluid challenge predicts fluid responsiveness during spontaneous breathing under spinal anaesthesia: An observational study. Eur J Anaesthesiol. 2015;32(9):645–9.
Guinot PG, de Broca B, Bernard E, Abou Arab O, Lorne E, Dupont H. Respiratory stroke volume variation assessed by oesophageal Doppler monitoring predicts fluid responsiveness during laparoscopy. Br J Anaesth. 2014;112(4):660–4.
Guinot PG, Godart J, de Broca B, Bernard E, Lorne E, Dupont H. End-expiratory occlusion manoeuvre does not accurately predict fluid responsiveness in the operating theatre. Br J Anaesth. 2014;112(6):1050–4.
Heenen S, De Backer D, Vincent JL. How can the response to volume expansion in patients with spontaneous respiratory movements be predicted? Crit Care. 2006;10(4):R102.
L’Hermite J, Muller L, Cuvillon P, Bousquet PJ, Lefrant JY, de La Coussaye JE, Ripart J. Stroke volume optimization after anaesthetic induction: An open randomized controlled trial comparing 0.9% NaCl versus 6% hydroxyethyl starch 130/0.4. Ann Fr Anesth Reanim. 2013;32(10):e121–127.
Hong DM, Lee JM, Seo JH, Min JJ, Jeon Y, Bahk JH. Pulse pressure variation to predict fluid responsiveness in spontaneously breathing patients: tidal vs. forced inspiratory breathing. Anaesthesia. 2014;69(7):717–22.
Huang CC, Fu JY, Hu HC, Kao KC, Chen NH, Hsieh MJ, Tsai YH. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med. 2008;36(10):2810–6.
Jung SM, Ryu S, Cho YC, Lee SH, Lim JS, Yun SY, Yoo IS. Validity of Corrected Flow Time (FTc) as a Predictor of Fluid Responsiveness in Patients with Sepsis-induced Hypotension. J Kor Soc Emerg Med. 2012;23(1):56–61.
Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29(2):64–9.
Kuiper AN, Trof RJ, Groeneveld AB. Mixed venous O2 saturation and fluid responsiveness after cardiac or major vascular surgery. J Cardiothorac Surg. 2013;8:189.
Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, Monnet X. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961–6.
Lakhal K, Ehrmann S, Benzekri-Lefevre D, Runge I, Legras A, Dequin PF, Mercier E, Wolff M, Regnier B, Boulain T. Brachial cuff measurements of blood pressure during passive leg raising for fluid responsiveness prediction. Ann Fr Anesth Reanim. 2012;31(5):e67–72.
Lakhal K, Ehrmann S, Perrotin D, Wolff M, Boulain T. Fluid challenge: tracking changes in cardiac output with blood pressure monitoring (invasive or non-invasive). Intensive Care Med. 2013;39(11):1953–62.
Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125–32.
Lanspa MJ, Brown SM, Hirshberg EL, Jones JP, Grissom CK. Central venous pressure and shock index predict lack of hemodynamic response to volume expansion in septic shock: a prospective, observational study. J Crit Care. 2012;27(6):609–15.
Lee JH, Kim JT, Yoon SZ, Lim YJ, Jeon Y, Bahk JH, Kim CS. Evaluation of corrected flow time in oesophageal Doppler as a predictor of fluid responsiveness. Br J Anaesth. 2007;99(3):343–8.
Loupec T, Nanadoumgar H, Frasca D, Petitpas F, Laksiri L, Baudouin D, Debaene B, Dahyot-Fizelier C, Mimoz O. Pleth variability index predicts fluid responsiveness in critically ill patients. Crit Care Med. 2011;39(2):294–9.
Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med. 2011;26(2):116–24.
Mahjoub Y, Pila C, Friggeri A, Zogheib E, Lobjoie E, Tinturier F, Galy C, Slama M, Dupont H. Assessing fluid responsiveness in critically ill patients: False-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med. 2009;37(9):2570–5.
Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007;33(7):1133–8.
Mallat J, Meddour M, Durville E, Lemyze M, Pepy F, Temime J, Vangrunderbeeck N, Tronchon L, Thevenin D, Tavernier B. Decrease in pulse pressure and stroke volume variations after mini-fluid challenge accurately predicts fluid responsivenessdagger. Br J Anaesth. 2015;115(3):449–56.
Mekontso-Dessap A, Tual L, Kirsch M, D’Honneur G, Loisance D, Brochard L, Teboul JL. B-type natriuretic peptide to assess haemodynamic status after cardiac surgery. Br J Anaesth. 2006;97(6):777–82.
Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Arterial pressure changes during the Valsalva maneuver to predict fluid responsiveness in spontaneously breathing patients. Intensive Care Med. 2009;35(1):77–84.
Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Brachial artery peak velocity variation to predict fluid responsiveness in mechanically ventilated patients. Crit Care. 2009;13(5):R142.
Monnet X, Letierce A, Hamzaoui O, Chemla D, Anguel N, Osman D, Richard C, Teboul JL. Arterial pressure allows monitoring the changes in cardiac output induced by volume expansion but not by norepinephrine. Crit Care Med. 2011;39(6):1394–9.
Monnet X, Picard F, Lidzborski E, Mesnil M, Duranteau J, Richard C, Teboul JL. The estimation of cardiac output by the Nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care. 2012;16(5):R212.
Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, Persichini R, Anguel N, Richard C, Teboul JL. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41(6):1412–20.
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402–7.
Monnet X, Dres M, Ferre A, Le Teuff G, Jozwiak M, Bleibtreu A, Le Deley MC, Chemla D, Richard C, Teboul JL. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth. 2012;109(3):330–8.
Monnet X, Bleibtreu A, Ferre A, Dres M, Gharbi R, Richard C, Teboul JL. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40(1):152–7.
Moretti R, Pizzi B. Inferior vena cava distensibility as a predictor of fluid responsiveness in patients with subarachnoid hemorrhage. Neurocrit Care. 2010;13(1):3–9.
Muller L, Louart G, Bousquet PJ, Candela D, Zoric L, de La Coussaye JE, Jaber S, Lefrant JY. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010;36(3):496–503.
Natalini G, Rosano A, Taranto M, Faggian B, Vittorielli E, Bernardini A. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: a clinical trial. Anesth Analg. 2006;103(6):1478–84.
Oliveira-Costa CD, Friedman G, Vieira SR, Fialkow L. Pulse pressure variation and prediction of fluid responsiveness in patients ventilated with low tidal volumes. Clinics (Sao Paulo). 2012;67(7):773–8.
Perner A, Faber T. Stroke volume variation does not predict fluid responsiveness in patients with septic shock on pressure support ventilation. Acta Anaesthesiol Scand. 2006;50(9):1068–73.
Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38(3):422–8.
Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013;39(4):612–9.
Preau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38(3):819–25.
Royer P, Bendjelid K, Valentino R, Resiere D, Chabartier C, Mehdaoui H. Influence of intra-abdominal pressure on the specificity of pulse pressure variations to predict fluid responsiveness. J Trauma Acute Care Surg. 2015;78(5):994–9.
Saugel B, Kirsche SV, Hapfelmeier A, Phillip V, Schultheiss C, Schmid RM, Huber W. Prediction of fluid responsiveness in patients admitted to the medical intensive care unit. J Crit Care. 2013;28(4):537.e531–539.
Siswojo AS, Wong DM, Phan TD, Kluger R. Pleth variability index predicts fluid responsiveness in mechanically ventilated adults during general anesthesia for noncardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(6):1505–9.
Smorenberg A, Lust EJ, Beishuizen A, Meijer JH, Verdaasdonk RM, Groeneveld AB. Systolic time intervals vs invasive predictors of fluid responsiveness after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2013;44(5):891–7.
Soltner C, Dantec R, Lebreton F, Huntzinger J, Beydon L. Changes in R-Wave amplitude in DII lead is less sensitive than pulse pressure variation to detect changes in stroke volume after fluid challenge in ICU patients postoperatively to cardiac surgery. J Clin Monit Comput. 2010;24(2):133–9.
Song Y, Kwak YL, Song JW, Kim YJ, Shim JK. Respirophasic carotid artery peak velocity variation as a predictor of fluid responsiveness in mechanically ventilated patients with coronary artery disease. Br J Anaesth. 2014;113(1):61–6.
Sturgess DJ, Pascoe RL, Scalia G, Venkatesh B. A comparison of transcutaneous Doppler corrected flow time, b-type natriuretic peptide and central venous pressure as predictors of fluid responsiveness in septic shock: a preliminary evaluation. Anaesth Intensive Care. 2010;38(2):336–41.
Suehiro K, Rinka H, Ishikawa J, Fuke A, Arimoto H, Miyaichi T. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing airway pressure release ventilation. Anaesth Intensive Care. 2012;40(5):767–72.
Taton O, Fagnoul D, De Backer D, Vincent JL. Evaluation of cardiac output in intensive care using a non-invasive arterial pulse contour technique (Nexfin((R))) compared with echocardiography. Anaesthesia. 2013;68(9):917–23.
Vallee F, Fourcade O, De Soyres O, Angles O, Sanchez-Verlaan P, Pillard F, Smail N, Olivier M, Genestal M, Samii K. Stroke output variations calculated by esophageal Doppler is a reliable predictor of fluid response. Intensive Care Med. 2005;31(10):1388–93.
Vallee F, Richard JC, Mari A, Gallas T, Arsac E, Verlaan PS, Chousterman B, Samii K, Genestal M, Fourcade O. Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med. 2009;35(6):1004–10.
van Haren FM, Sleigh J, Boerma EC, La Pine M, Bahr M, Pickkers P, van der Hoeven JG. Hypertonic fluid administration in patients with septic shock: a prospective randomized controlled pilot study. Shock. 2012;37(3):268–75.
Yazigi A, Khoury E, Hlais S, Madi-Jebara S, Haddad F, Hayek G, Jabbour K. Pulse pressure variation predicts fluid responsiveness in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2012;26(3):387–90.
Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30(9):1734–9.
Vistisen ST, Struijk JJ, Larsson A. Automated pre-ejection period variation indexed to tidal volume predicts fluid responsiveness after cardiac surgery. Acta Anaesthesiol Scand. 2009;53(4):534–42.
Wiesenack C, Fiegl C, Keyser A, Prasser C, Keyl C. Assessment of fluid responsiveness in mechanically ventilated cardiac surgical patients. Eur J Anaesthesiol. 2005;22(9):658–65.
Wiesenack C, Fiegl C, Keyser A, Laule S, Prasser C, Keyl C. Continuously assessed right ventricular end-diastolic volume as a marker of cardiac preload and fluid responsiveness in mechanically ventilated cardiac surgical patients. Crit Care. 2005;9(3):R226–233.
Wilkman E, Kuitunen A, Pettila V, Varpula M. Fluid responsiveness predicted by elevation of PEEP in patients with septic shock. Acta Anaesthesiol Scand. 2014;58(1):27–35.
Xiao-ting W, Hua Z, Da-wei L, Hong-min Z, Huai-wu H, Yun L, Wen-zhao C. Changes in end-tidal CO2 could predict fluid responsiveness in the passive leg raising test but not in the mini-fluid challenge test: A prospective and observational study. J Crit Care. 2015;30(5):1061–6.
Zimmermann M, Feibicke T, Keyl C, Prasser C, Moritz S, Graf BM, Wiesenack C. Accuracy of stroke volume variation compared with pleth variability index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol. 2010;27(6):555–61.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets generated and analysed during the present study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
LT, DA and DB made substantial contributions to the design, acquisition and interpretation of data and drafting of the manuscript. HDA and MC made substantial contributions to study conception and design as well as analysis and interpretation of data. HDA, XW, NA, AR and MC were involved in revising the manuscript critically for important intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval or patient consent was necessary for the present study.
Consent for publication
Not applicable.
Competing interests
HDA received financial support from LiDCO for educational programs and for attending symposia. AR has received honoraria for serving on an advisory board for LiDCO, as well as honoraria from Covidien, Edwards Lifesciences and Cheetah. MC has received honoraria for speaking at symposia, financial support for educational programs and honoraria for serving on an advisory board from Edwards Lifesciences, LiDCO, Deltex, Massimo, BMEYE, Cheetah and ImaCor. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Study protocol, PRISMA checklist and additional figures. (PDF 674 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Toscani, L., Aya, H.D., Antonakaki, D. et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care 21, 207 (2017). https://doi.org/10.1186/s13054-017-1796-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-017-1796-9