Abstract
Objective
Assessing pulse pressure variation (PPV) to predict fluid responsiveness in mechanically ventilated patients with tidal volume (VT) and the impact of VT and airway driving pressure (Pplat − PEEP) on the ability of PPV for predicting fluid responsiveness.
Design
Prospective interventional study.
Setting
ICU of a university hospital.
Patients
Fifty-seven mechanically ventilated and sedated patients with acute circulatory failure requiring cardiac output (CO) measurement.
Intervention
Fluid challenge was given in patients with signs of hypoperfusion (oliguria <0.5 ml kg−1 h−1, attempt to decrease vasopressor infusion rate). Fluid responsiveness was defined as an increase in the stroke index (SI) ≥15%. Receiver-operating characteristic (ROC) curves were generated for PPV and central venous pressure (CVP).
Results
The stroke index was increased ≥15% in 41 patients (71%). At baseline, CVP was lower and PPV was higher in responders. The areas under the ROC curves of PPV and CVP were 0.77 (95% CI 0.65–0.90) and 0.76 (95% CI 0.64–0.89), respectively (P = 0.93). The best cutoff values of PPV and CVP were 7% and 9 mmHg, respectively. In 30 out of 41 responders, PPV was <13%. Using a polytomic logistic regression (Pplat − PEEP) was the sole independent factor associated with a PPV value <13% in responders. In these responders, (Pplat − PEEP) was ≤20 cmH2O.
Conclusion
In patients mechanically ventilated with low VT, PPV values <13% do not rule out fluid responsiveness, especially when (Pplat − PEEP) is ≤20 cmH2O.
Similar content being viewed by others
Introduction
Volume expansion remains a cornerstone of the management of patients with acute circulatory failure [1–3]. However, an excessive fluid loading could induce peripheral and pulmonary edema, compromising microvascular perfusion and oxygen delivery [4, 5]. Therefore, predicting fluid responsiveness could be of particular interest at the patient’s bedside. In the last decade, dynamic variables such as systolic pressure variation, stroke volume variation (SVV), respiratory variation in aortic blood flow monitored with esophageal Doppler and pulsed pressure variation (PPV) have been shown to be more accurate in predicting fluid responsiveness than classically used static parameters [central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP)] in mechanically ventilated patients without spontaneous breathing [6–15]. Because PPV is generated by the pressure transmitted from the airways to the pleural and pericardial spaces, it can be theoretically decreased when a low tidal volume (VT) is applied and/or in patients with low pulmonary compliance [16]. In 21 patients with hemodynamic instability, PPV has been shown to increase with the level of VT [17]. In patients with acute respiratory distress syndrome (ARDS), De Backer et al. [18] showed that the ability of PPV to predict fluid responsiveness was decreased when a tidal volume <8 ml kg−1 was applied. As the use of low VT (6–8 ml kg−1 of ideal body weight) has been shown to improve patient outcome in acute respiratory distress syndrome [19] and is deemed to prevent the occurrence of ARDS in mechanically ventilated patients, the predictive value of PPV in predicting fluid responsiveness could be questioned when low VT is applied [20, 21]. Therefore, the primary goal of the present study was focused on the ability of PPV to predict fluid responsiveness in sedated patients with acute circulatory failure and mechanically ventilated with low VT. Because PPV could theoretically vary according the VT and the lung compliance, the secondary goal was to assess the impact of VT and airway driving pressure on the ability of PPV to predict fluid responsiveness.
Materials and methods
The present study was approved by the Institutional Review Board (number 08/05-01) as it did not alter or delay the patients’ management. The patient’s closest contacts were informed of the study.
The sedated (midazolam or propofol with sufentanil or remifentanil with a targeted goal of sedation; Ramsay score [22] = 4–6) and mechanically ventilated ICU patients without spontaneous breathing [no spontaneous breath on the screen of the ventilator (Drager Evita 2 Dura, Germany), six patients with a Ramsay score = 6 were given neuromuscular blockade] with acute circulatory failure were eligible to participate in the study. Acute circulatory failure was defined as systolic blood pressure <90 mmHg or the need for vasopressors (dopamine >5 μg kg−1 min−1 or epinephrine or norepinephrine >0.1 μg kg−1 min−1) to maintain a systolic blood pressure >90 mmHg [7]. The association of a clinical infection, the presence of systemic inflammatory response syndrome and acute circulatory failure defined septic shock [23].
Inclusion and exclusion criteria
In mechanically ventilated and sedated ICU patients with acute circulatory failure, the following inclusion criteria were required:
-
The cardiac output or index (CO or CI) was monitored (pulmonary artery catheter Swan-Ganz CCOmbo CCO/SvO2, Edwards Lifesciences LLL, Irvine, CA, or PiCCO, Pulsion Medical Systems AG, Munchen, Germany).
-
A fluid challenge was indicated because of signs of hypoperfusion (oliguria <0.5 ml kg−1 h−1, CI inadequate for tissue needs, attempt to decrease vasopressor infusion rate).
Patients with cardiac arrhythmias, with known tricuspid insufficiency, with obvious impaired ventricular dysfunction (radiological evidence of cardiogenic pulmonary edema, PAOP >18 mmHg) were excluded. Moribund patients, parturient and patients <18 years of age were also excluded.
Fluid challenge procedure and fluid challenge responsiveness
The fluid challenge was given intravenously via a specific venous line at a constant rate (999 ml h−1, Fresenius Vial®, France, using an infusion pump with 250 or 500 ml isotonic saline or hydroxy-ethyl–starch solution 6% 130/0.4) according to the assessment of the attending physician [7]. The fluid responsiveness was defined as an increase in the stoke index (SI, ratio of CI and heart rate) ≥15%, separating the studied population into responders (R) and non-responders (NR) [7].
Measured variables and time of measurement
-
Patients characteristics: age, sex, height, weight and APACHE II score [24] at admission were recorded. The cause of acute circulatory failure, the inotropic and/or vasopressive support [epinephrine, norepinephrine, dobutamine, dopamine (μg kg−1 min−1)] and the number of organ dysfunctions using the ODIN score [25] were recorded.
-
Mechanical ventilation parameters: tidal volume (ml kg−1 of ideal body weight), respiratory rate (RR, cycle min−1), inspiratory oxygen fraction (FiO2), the level of positive end expiratory pressure (PEEP) and plateau pressures (cmH2O) were recorded. The ideal body weight was defined as follows: IBW = X + 0.91 [height (cm) −152.4] (X = 50 for male and X = 45.5 for female).
-
Hemodynamic parameters: heart rate (HR, bpm), mean arterial pressure (MAP, mmHg), central venous pressure (CVP, mmHg), PPV (%), CI (l min−1 m−2) and SI (ml m−2) were measured or calculated before fluid challenge (baseline = T 0) and within 10 min after the end of fluid challenge (T 1). The ratio HR/RR was calculated as De Backer recently showed that this ratio could impact the reliability of PPV to predict fluid responsiveness in hypovolemic patients [26]. The CO was calculated by the mean of three measurements using transpulmonary thermodilution in patients with PiCCO (injection of 15 ml of cold saline with an adequate thermodilution curve on the monitor screen) or by continuous thermodilution in patients with a pulmonary artery catheter. The CVP and MAP were measured invasively with a zero referenced to the middle axillary line. The CVP was measured at end-expiration. The PPV value was calculated as initially reported by Michard et al. [7], using the recording of invasive arterial pressure on the monitor screen (Intellivue MP 160, Philips, Eindhoven, The Netherlands). Maximal (PPmax) and minimal pulse pressures (PPmin) were calculated as described by Michard et al. [7]. The pulse pressure variation (PPV %) was calculated as follows: PPV = 100 × 2[(PPmax − PPmin)/(PPmax + PPmin)]. PPV was evaluated in triplicate over each of three consecutive respiratory cycles. The mean values of the three determinations were used for statistical analysis.
Statistical analysis: data are expressed as the median with 5th and 95th percentiles. Comparison of two medians was performed using the Mann–Whitney test and comparison of two proportions using the Fisher exact method. Receiver-operator characteristic (ROC) curves were constructed to evaluate the capacity of PPV and CVP to predict fluid responsiveness. When the ROC curve area was greater than 0.5, the best cutoff value was defined by the Youden index. The comparison between the ROC curve of PPV and CVP was performed using the Hanley test [27].
Considering that the areas under the ROC curves (AUC) reported by Michard et al. [7] (0.98 ± 0.03), Vieillard Baron et al. [11] (0.940 ± 0.038) and Kramer et al. [10] [0.99 (0.96–100)], we assumed an area under the ROC curve of PPV should be >0.87 (the lowest value previously observed) to be clinically relevant. To observe an AUC equal to 0.92 ± 0.05, 55 patients had to be included in the study. Analysis was performed using SAS version 8.1, SAS Institute, Cary, NC, and a P value <0.05 was considered statistically significant.
Results
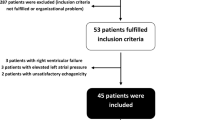
Eighty-three patients (57 men) were eligible for the study. Twenty-six were excluded because of cardiac arrhythmia (n = 16), moribund patients (n = 5), violation of the study protocol (n = 3), right-to-left shunt (n = 1) or risk of pulmonary edema (PAOP = 25 mmHg) (n = 1). Therefore, 57 patients [18 women (32%); septic shock = 39 patients (68%), hemorrhagic shock = 9 patients (16%), vasoplegic shock with systemic inflammatory response syndrome = 9 patients (16%)] (Table 1) were included. A VT <8 ml kg−1 IBW was applied in 54 out of 57 included patients [95% of studied population; median value = 5.5 ml kg−1 IBW (3.5–7.7)]. Fluid challenge induced an SI increase ≥15% in 41 patients (72%) (R). Table 2 shows the comparison between R and NR. At baseline, MAP, CVP and SI were significantly lower and PPV was significantly higher in R than in NR (Table 2). For the other studied parameters, there was no statistical difference. Baseline individual values of CVP and PPV of R and NR are shown in Fig. 1. The areas under the ROC curves of PPV and CVP were 0.77 (95% CI 0.65–0.90) and 0.76 (95% CI 0.64–0.89), respectively (P = 0.93) (Fig. 2). The best cutoff values of PPV and CVP were 7% [sensitivity = 61% (48–74), specificity = 94% (70–99), positive predictive value = 96% (91–100), negative predictive value = 52% (39–65), accuracy = 77%] and 9 mmHg [sensitivity = 68% (52–82), specificity = 81% (54–96), positive predictive value = 90% (82–98), negative predictive value = 50% (37–63), accuracy 74%], respectively.
Figure 1 shows that 17 Rs were associated with low PPV values (<7%, the best cutoff value). Thus, a post-hoc analysis was performed to compare Rs with PPV values <7%, with PPV values between 7 and 12% and with PPV values ≥13%. The tidal volume (in ml and in ml kg−1 of IBW), the baseline heart rate, the ratio RR/HR and the airway driving pressure (Pplat − PEEP) were higher in Rs with PPV ≥13%, whereas SIs were lower in Rs ≥13% (Table 3). Using a polytomic logistic regression (stepwise), the airway driving pressure (Pplat − PEEP) was the sole independent factor associated with a PPV value <13% in Rs [PPV ≥13% reference; 13 < PPV ≤7%, odds ratio (OR) = 0.61 (0.44–0.85); PPV <7%, OR = 0.67 (0.50–0.90)]. Figure 3 shows that the driving pressure was ≤20 cmH2O in responders with PPV <13%. In responders with PPV >13%, HR/RR was statistically higher than in other responders [5.88 vs. 4.35 (PPV between 7 and 13%) and 3.57 (PPV < 7%), P < 0.001].
Discussion
The present study including 57 patients with acute circulatory failure challenges the ability of PPV to predict the fluid responsiveness. Whereas a PPV value ≥13% accurately predicts a fluid responsiveness, an increase in SI ≥15% could be observed in 13/14 patients with PPVs between 7 and 12% and in 17 patients with a PPV value <7%, especially when an airway driving pressure ≤20 cmH2O was applied.
This study involved patients with all types of shock for whom fluid administration remains one of the main therapeutic treatments for hypotension. The inclusion of nine patients with hemorrhagic shock (all responders) could explain 72% of responders, which is in the high range of what has been previously reported (40–72%), and the high value of the AUC of CVP [8].
The present study shows an area under the ROC curve of PPV = 0.77 (95% CI 0.65–0.90), which is far from those reported by Michard [7], Vieillard-Baron and Kramer (AUC of ROC curve 0.98, 0.94 and 0.99, respectively) [7, 10, 11]. However, our findings are closer to those reported by Hofer [12], De Backer [18] and Huang [28] (0.808, 0.76 and 0.768, respectively). The best cutoff value was 7% with a sensitivity and specificity, positive and negative predictive values, and accuracy of 61, 94, 88, 96 and 50%, respectively. This finding means that a positive fluid responsiveness could be associated with low value of PPV. As shown in the Fig. 1, the findings of the present study mean that a PPV value ≥13% and even ≥7% is highly predictive of fluid responsiveness, but fluid responsiveness could not be ruled out in patients with lower PPV values.
The use of a low VT (<8 ml kg−1 IBW in 54 out of 57 patients) is probably the main explanation of the discrepancy between the findings of the present study and those of the previous ones. Pulsed pressure variation is caused by the transmission of airway pressure to the pleural and pericardial spaces, which induces changes in venous return and cardiac preload. Therefore, PPV could be theoretically limited when the part of transmitted airway pressure to the pleural and pericardial spaces is low. This could be due to the use of low VT in normal lungs with high compliance and in ARDS patients with lungs that have low compliance and are stiff. In these conditions, the probability of transmitting a sufficient pressure variation to the pleural and pericardial spaces to induce large PPV is low [16, 29, 30]. In anesthetized pigs, Reuter et al. [31] and Kim et al. [32] have shown that the higher the tidal volume is, the higher variation of stroke volume or PPV, respectively. In 21 critically ill patients, Charron et al. [17] showed that PPV increases with the increase in VT. In septic patients with ARDS/ALI mechanically ventilated patients with VT <8 ml kg−1 IBW, De Backer et al. [18] reported a lower ability of PPV (area of the ROC curve of PPV = 0.71 ± 0.09) to predict fluid responsiveness. This finding was recently confirmed by Vallée et al. [33], who reported that PPV failed to accurately predict fluid responsiveness in patients ventilated with VT <8 ml kg−1 [ROC curve area = 0.62 (0.45–0.80)]. Huang et al. [28] also reported an AUC for the ROC curve of PPV = 0.768 in early ARDS patients who were ventilated with VT = 6.4 ± 0.7 ml kg−1 IBW. The present study reporting patients in acute circulatory failure and mechanically ventilated with low VT shows a similar value of AUC of the ROC curve [0.77 (95% CI 0.65–0.90)]. In the studies reported by Michard et al. [7], Vieillard Baron et al. [11] and Kramer et al. [10], respectively, greater VT was used (8–12 ml kg−1; 8 ± 2 ml kg−1 with a respiratory rate 15 breaths/min−1; 8–10 ml kg−1, respectively). When a low tidal volume is applied in a patient with normal lung, the mechanical ventilation induces a slight alteration in driving pressure. In ARDS patients with a high lung stiffness, the application of low VT leads to higher driving pressures that are transmitted less to the cardiac cavities. In both cases, the impact of mechanical ventilation on venous return and thus on PPV is blunted. The lack of transmission of airway pressure to the intracardiac cavities could also explain the lack of prediction of fluid responsiveness by dynamic preload parameters (PPV and stroke volume variation) in open-chest conditions [34]. Moreover, the use of low VT could lead to using higher respiratory rates and decreasing the HR/RR ratio in order to prevent severe hypercapnia. Recently, De Backer et al. [26] reported that the increase in RR decreased the PPV in 17 hypovolemic patients. In this study, PPV became negligible when HR/RR decreased below 3.6, which is close to the ratio found in responders with PPV <7% in our study. Finally, most of the patients were supported with norepinephrine, which has been shown to blunt the effect of mechanical ventilation on PPV [35].
Despite different findings, the present study should not be understood as challenging the ability of PPV to predict fluid responsiveness. Indeed, a PPV value ≥7% has a positive predictive value = 96%, meaning that high PPV values (>7%) were associated with fluid responsiveness. The use of VT <8 ml kg−1 IBW could be suggested to decreases the threshold to predict responsiveness and to enlarge the overlap between responders and non-responders (classically called the grey zone). In seven anesthetized and mechanically ventilated dogs, Kim and Pinsky [32] recently showed that PPV decreases from 20.1 ± 10.8 to 9.5 ± 5.4% when VT decreases from 20 to 5 ml kg−1.
In the present study, several limitations could be advanced. Firstly, the fluid challenge was not standardized, but was achieved according to the risk/benefit ratio of fluid challenge assessed by the attending physician. There is no consensus concerning the product and the volume of fluid challenge [36]. Secondly, the use of triplicate injections for CO measurement could increase the amount of fluid challenge. These first two limitations could lead to giving a greater amount of fluid, shifting a non-responder to being a responder. However, the main message of the present study remains similar: a low PPV value does not rule out the efficacy of fluid challenge, and the threshold of PPV to discriminate R from NR could be decreased less than 10%. Thirdly, PPV was measured using a manual method as initially reported by Michard et al. [7]. A recent study demonstrated that sampling duration alters pulse pressure variation [32]. Therefore, we cannot assume that the same findings would be reported if PPV was automatically measured and recorded. Fourthly and importantly, even if a PPV value <13% in responders was always associated with a driving pressure ≤20 mmHg, some responders with PPV >13% showed an airways driving pressure <20 mmHg. This limitation could challenge the findings of the present study. However, the main result is that a low PPV value could be associated with fluid responsiveness, especially in patients in whom the transmission of pleural pressure to the pericardial space could be compromised, such as in ALI and ARDS.
In daily clinical practice, the present study confirms the predictive values of a high PPV. However, a PPV <13% cannot rule out fluid responsiveness, especially when pulmonary driving pressure ≤20 cmH2O is applied in mechanically ventilated patients, corresponding to tidal volume <8 ml kg−1.
References
Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, Meduri GU, Moreno RP, Putensen C, Stewart T, Torres A (2007) Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27–28 April 2006. Intensive Care Med 33:575–590
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Wang P, Zhou M, Rana MW, Ba ZF, Chaudry IH (1992) Differential alterations in microvascular perfusion in various organs during early and late sepsis. Am J Physiol 263:G38–G43
Ferguson ND, Meade MO, Hallett DC, Stewart TE (2002) High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med 28:1073–1077
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P (1998) Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology 89:1313–1321
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Coriat P, Vrillon M, Perel A, Baron JF, Le Bret F, Saada M, Viars P (1994) A comparison of systolic blood pressure variations and echocardiographic estimates of end-diastolic left ventricular size in patients after aortic surgery. Anesth Analg 78:46–53
Kramer A, Zygun D, Hawes H, Easton P, Ferland A (2004) Pulse pressure variation predicts fluid responsiveness following coronary artery bypass surgery. Chest 126:1563–1568
Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F (2004) Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 30:1734–1739
Hofer CK, Muller SM, Furrer L, Klaghofer R, Genoni M, Zollinger A (2005) Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest 128:848–854
Auler JO Jr, Galas F, Hajjar L, Santos L, Carvalho T, Michard F (2008) Online monitoring of pulse pressure variation to guide fluid therapy after cardiac surgery. Anesth Analg 106:1201–1206 table of contents
Cannesson M, Slieker J, Desebbe O, Bauer C, Chiari P, Henaine R, Lehot JJ (2008) The ability of a novel algorithm for automatic estimation of the respiratory variations in arterial pulse pressure to monitor fluid responsiveness in the operating room. Anesth Analg 106:1195–1200 table of contents
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2005) Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med 31:1195–1201
Michard F (2005) Changes in arterial pressure during mechanical ventilation. Anesthesiology 103:419–428 quiz 449-415
Charron C, Fessenmeyer C, Cosson C, Mazoit JX, Hebert JL, Benhamou D, Edouard AR (2006) The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg 102:1511–1517
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL (2005) Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 31:517–523
(2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A (2005) Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med 31:922–926
Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, van der Poll T, Vroom MB, Hollmann M, Schultz MJ (2008) Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 108:46–54
Ramsay MA, Savege TM, Simpson BR, Goodwin R (1974) Controlled sedation with alphaxalone–alphadolone. Br Med J 2:656–659
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Fagon JY, Chastre J, Novara A, Medioni P, Gibert C (1993) Characterization of intensive care unit patients using a model based on the presence or absence of organ dysfunctions and/or infection: the ODIN model. Intensive Care Med 19:137–144
De Backer D, Taccone FS, Holsten R, Ibrahimi F, Vincent JL (2009) Influence of respiratory rate on stroke volume variation in mechanically ventilated patients. Anesthesiology 110:1092–1097
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Huang CC, Fu JY, Hu HC, Kao KC, Chen NH, Hsieh MJ, Tsai YH (2008) Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med 36:2810–2816
Jardin F, Genevray B, Brun-Ney D, Bourdarias JP (1985) Influence of lung and chest wall compliances on transmission of airway pressure to the pleural space in critically ill patients. Chest 88:653–658
Lefrant JY, De Backer D (2009) Can we use pulse pressure variations to predict fluid responsiveness in patients with ARDS? Intensive Care Med 35(6):966–968
Reuter DA, Bayerlein J, Goepfert MS, Weis FC, Kilger E, Lamm P, Goetz AE (2003) Influence of tidal volume on left ventricular stroke volume variation measured by pulse contour analysis in mechanically ventilated patients. Intensive Care Med 29:476–480
Kim HK, Pinsky MR (2008) Effect of tidal volume, sampling duration, and cardiac contractility on pulse pressure and stroke volume variation during positive-pressure ventilation. Crit Care Med 36:2858–2862
Vallee F, Richard JC, Mari A, Gallas T, Arsac E, Verlaan PS, Chousterman B, Samii K, Genestal M, Fourcade O (2009) Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med 35(6):1004–1110
de Waal EE, Rex S, Kruitwagen CL, Kalkman CJ, Buhre WF (2009) Dynamic preload indicators fail to predict fluid responsiveness in open-chest conditions. Crit Care Med 37:510–515
Nouira S, Elatrous S, Dimassi S, Besbes L, Boukef R, Mohamed B, Abroug F (2005) Effects of norepinephrine on static and dynamic preload indicators in experimental hemorrhagic shock. Crit Care Med 33:2339–2343
Vincent JL, Gerlach H (2004) Fluid resuscitation in severe sepsis and septic shock: an evidence-based review. Crit Care Med 32:S451–S454
Acknowledgments
The authors thank Prof. Jean Louis Teboul and Prof. Daniel De Backer for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muller, L., Louart, G., Bousquet, PJ. et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med 36, 496–503 (2010). https://doi.org/10.1007/s00134-009-1686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1686-y