Abstract
Attention has recently increased in the application of electrospun fibers because of their putative capability to create nanoscale platforms toward tissue engineering. To some extent, electrospun fibers are applicable to the extracellular matrix by providing a three-dimensional microenvironment in which cells could easily acquire definite functional shape and maintain the cell-to-cell connection. It is noteworthy to declare that placement in different electrospun substrates with appropriate physicochemical properties enables cells to promote their bioactivities, dynamics growth and differentiation, leading to suitable restorative effects. This review paper aims to highlight the application of biomaterials in engineered vascular grafts by using electrospun nanofibers to promote angiogenesis and neovascularization
Similar content being viewed by others
Introduction
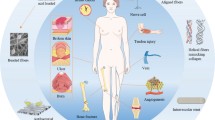
Cardiovascular disease is one of the most important issues contributes to human death globally [1]. In cardiovascular medicine, surgical approach and transplantation are often desired therapeutic options to care for patients with pathological conditions [2]. Peripheral vascular disease is touted as a common circulatory disorder that reduces blood nourishment and ultimately leads to the ischemic condition [3]. In the circumstances with the complete vessels obstruction, vascular transplantation and bypass surgery are highly recommended [4]. At the moment, vascular grafts are currently prepared by vascular sections of the patient’s body or from an appropriate donor [5]. Calling attention, vascular transplantation is not often sufficient to meet patient needs due to limited vascular resources. Also, the risk of thrombosis, infection, and rejection of the vascular transplant are very likely in these conditions [6,7,8]. In line with these conditions, the need for transplantation of artificial vessel is felt more than ever. In this regard, novel tissue engineering technology can be used for vascular reconstruction. So far, the vascular tissue engineering for large vessels had promising successes for vessels structure with a diameter of 6 mm, but the reconstruction of structures below this value is faced with several challenges. The provision of tubes with suitable resistance to the pressure and cyclic loading of blood that couple with the host vessels possessing antithrombotic activity are at the center of attention [9, 10]. Tissue engineering is interdisciplinary scientific modalities uses different methodologies to circumvent problems associated with tissue injury or organ loss. In terms of structural components, tissue engineering consists of three main components as follows; scaffolds act as an underlying substitute with an applicable function to the ECM, growth factors that promote intracellular signaling effectors and distinct cell types as the main component of the tissue structure (Fig. 1) [11]. To achieve this end, tissue engineering usually starts with the fabrication of a specific matrix composed of multiple suitable components from a natural source (proteins) and synthetic materials (polymers). The constructs should have potential to imitate almost all aspects of the natural microenvironment. However, scientific challenges still exist in the fabricated substrates parameters and values associated with the creation of environment comparable to in vivo condition. Up to the present, the application of electrospun-nanofibers, as a scaffold, is being popular in tissue engineering. To fabricate electrospun-nanofibers, multiple nano-sized scaffolding can be designed in 2D, and 3D with a porous structure to attain a high surface area-to-volume ratio, allowing cells to maintain juxtacrine interaction with each other [12]. All of these features have contributed to the emergence of comprehensive high throughput restorative effects in which electrospun nanofibers have been suggested as a novel modality with satisfactory outcomes in the restoration of cutaneous tissue, blood vessels, cartilage, bone, etc. [13, 14].
The current review article tried to collect novel data in the fabrication of vascular grafts, in particular, the electrospinning approach, for the induction of angiogenesis and blood nourishment. We also scrutinized cellular response and clinical promise of engineered vascular grafts produced by electrospun nanofibers.
General blood vessels structure
In general, blood circulates through the body via closed different vessels such as arteries, veins, and capillaries. Arteries have the potential to transport blood from the cardiac tissue to other organs, plus the systemic arteries are transporting oxygen-rich blood to the tissues. Accordingly, the arteries need an elastic wall to counteract the pressure effected when the ventricular and muscle contraction to enable better contraction to aid move the blood [15, 16].
The veins are responsible for returning blood to the heart. Most often, the vein returns the deoxygenated blood from various tissues to the heart, with the exception of the pulmonary vein and the umbilical vein that returns oxygen-carrying blood to the heart. The vein is less muscular than the artery and has valves that prevent blood backflow [17]. The smallest and most abundant vessels in the body are capillaries that form the connections between the arteries and veins. Since the capillaries have a thin wall and the blood flow in them is slow, so the swapping of materials between blood and cells is done in the capillaries [18]. From a histological point of view, vessels contain three distinct zones from luminal to outer surface including tunica intima, tunica media and tunica adventitia (Fig. 2) [19]. A term lumen refers to the interior space of the vessel which is surrounded by the vessel wall. The layer tunica intima is arteries inner layer that paved with a single-layer column of ECs lining subendothelial layer and is in direct contact with blood flow. For the consistency and resistance of tunica intima against mechanical stress, the endothelial layer is supported by the basal internal elastic lamina. Internal elastic lamina acts as a border and isolates the tunica intima from the lower middle layer named tunica media. To be informed, tunica media is the middle layer of vessels wall consisted of a regular circle of rows of α-SMCs, fibroblasts surround by elastic fibers in collagenous bed. The outer layer, tunica adventitia, is composed of fibroblasts, collagen and distinguishable from tunica media by an external elastic lamina [20, 21]. In large size vessels with a thick wall, the penetration of blood substances is impossible while capillaries composed of a single-cell thin layer have the potential to exchange materials reciprocally between blood and neighboring tissues [9, 22, 23]. Based on the scientific reports, two forces interact with the exchange of materials through the capillary walls. As previously mentioned in the literature, blood pressure is high inside capillaries and thus provides pressure for the penetration of materials into the interstitial fluid. In contrast, the existence of osmotic force originated from by plasma proteins forces the transportation of liquid phase and metabolic byproducts from tissues (interstitial fluid) to the capillaries. Of note, the amount of this pressure is high in vessels context compared to the interstitial fluid [15, 24, 25]. The biomechanical and biophysical properties of the vessels are related to the entity of ECM enclosed vascular cells. Along with this issue, experiments have shown that cell differentiation, migration, and polarization are under the influence of ECM and the quality and quantity of cell surface glycoconjugates [26, 27]. Therefore, the existence of a unique ECM structure with prominent mechanical properties makes blood vessels suitable for circuit liquid phage. Such mechanical features such as elasticity, compressibility, tensile stiffness, and viscosity are provided by elastin, proteoglycans, and collagens [28].
Tissue engineering of blood vessels
Tissue engineering
In spite of human body vulnerability to injury, it has a unique strength and capacity for self-repair. Along with the development and progress of biomaterial engineering and regenerative modalities, the dream of tissue replacement seems achievable in the near future [29]. In 1900, Carell introduced the term “tissue engineering” for the first time. By virtue of the introduction of a de novo method for vascular anastomosis, the implication of modern tissue transplantation was raised [30, 31]. In short, tissue engineering means the in vitro development and modulation of distinct molecules and cells in natural and synthetic constructs with the purpose of replacing and repairing the damaged tissue. In this modality, porous materials are fabricated as suitable ECM and underlying substrates for the promotion of cell dynamic growth. To increase cellular adaption, various growth factors and cytokines could be conjugated to the substrates. In a better word, the concept of tissue engineering encompasses the spatial support of cells with a prominent physical feature in a 3D milieu. Commonly, the scaffolds mainly consist of synthetic or natural materials (collagen, elastin, and fibrin) acts as basal scaffolds, anchorages for cells or that cells can orient and acquire functional maturation for cultivation in a suitable niche [29, 32]. After approval of proper cell growth in the porous scaffolds, the constructs could be transplanted to the target sites in vivo. Angiogenesis develops into scaffolds to nourish the transplanted cells and to prohibit cell death [29, 33] (Fig. 3). Commensurate with these statements, the promotion of vascularization into the transplanted construct seems to be vital for successful tissue engineering and repair. Once the engineered scaffolds are transplanted into in vivo condition, direct contact of scaffolds with the microenvironment allows the recruitment of diverse ions, proteins, polysaccharides, enzymes and different types of cells as well. Early researches in the mid-twentieth century were focused on the development of bioconversion materials to result in minimal host response, inactive blood transport, and minimal interaction with neighboring tissues. To this end, numerous synthetic materials are extensively available, for example, Silicone and Teflon which not exactly applicable for medical proposes. Today, many biomaterials are designed to maintain the reciprocal interaction between proteins and cells at the molecular levels in a very precise and manageable manner. The main purpose of the development and design of these biomaterials is that the scaffolds should contain chemical or structural information that can imitate cell-cell interaction and control the formation of tissue. Agents such as growth factors and the adhesion Arginyl-glycyl-aspartic acid (RGD) peptide sequences and other molecules with a structure resembling ECM components are highly requested [34, 35].
Common biomaterials in tissue engineering
The term “biomaterial” means the technology of application materials as scaffolds with an ability to control cellular bioactivities. In addition to the comparable structure of biomaterials to the in vivo condition, these materials must be compatible with the host sites while at the same time stimulating cell signaling by providing extracellular signals. Williams described the term biomaterial as any synthetic or natural substances that include all or part of a living structure and function with the capability to replace with the host tissue (Table 1) [36, 37]. Biomaterial properties must be defined in accordance with the type of cells and organs and transplantation site. The importance of mechanical properties, which is directly related to the target tissue, should not be ignored [38]. Irrespective the specific feature of different scaffolds, all biomaterials should possess biocompatibility and eschew from the immune response [39]. Regarding engineered vessels grafts, thrombogenic properties of these structures should be investigated which are in close contact with blood and coagulation factors. In addition, it should be noted that the biomaterials used in these structures should have an inherent resistance to tolerate and adapt to continuous blood pressure. As a matter of fact, different values such as burst pressure, suture strength and exhaustion must be considered prior to implantation [39, 40].
In 1896, the first report of natural vascular transplantation by Jaboulay and Briau was done, although the vascular anastomoses rate was incomplete and thrombosis induced [41, 42]. To date, many artificial techniques have been developed for the fabrication of vascular autografts, which are routinely used in bypass surgery. However, the access of autografts has some limitations. By the discovery of Vinyon N (nylon) by Voorhees and Dacron® by DeBakey, the development of artificial vascular grafts entered a new phase [43]. Despite the fact that transplantation of larger vascular grafts with a diameter of > 6 mm) contributed to prominent high-flow rate, but the development of thrombi and increase of compliance mismatch have been shown in a small diameter vessel [44, 45]. To overcome these limitations, advanced techniques have been developed to increase construct potency, for example, chemical modifications on surfaces with different coating methods and cell plating approach.
Natural biomaterials have superior effects to synthetic counterparts due to the existence of adhesion motifs; however, synthetic materials have a higher mechanical resistance rate [46, 47]. Natural polymers such as collagen, alginate, fibronectin, chitosan, fibrin, and gelatin often provide the moieties for cell adhesion, proliferation and functional differentiation thus attracted a lot of attention. Due to lack of sufficient mechanical properties of natural polymers, it is not reasonable to use them for vascular scaffolds alone. To solve this issue, the combination of natural and synthetic polymers could be an effective strategy [48, 49]. The mixture must be able to provide all or some of the following conditions for cell growth dynamic and mimic in vivo condition.
Scaffold design
Each tissue consists of ECM and a certain number of cells. The ECM acts as a 3D scaffold to preserve cell-to-cell integrity inside the body. In general, three types of molecules are present in the ECM of all tissues as follows; (a) structural proteins such as collagen and elastin which provide flexibility and strength; (b) protein-polysaccharide complexes to integrate with the structural proteins (proteoglycans); and (c) adhesive glycoproteins such as fibronectin and laminin that connect each cell to the ECM [76, 77]. It is shown that cells interact with the scaffolds after plating on the surface [78]. In addition to the supportive role of the scaffold in maintaining cell adhesion, ECM serves as an appropriate reservoir of water, nutrients, cytokines and growth factors [79]. Meanwhile, the constructs must have good macroscopic and microscopic properties in which these properties not only affect the cell’s life, signaling pathways, growth, proliferation, or organization but also affect the expression of the gene and maintain the cell’s phenotype [80].
Critical factors in the design of engineered vascular tissue scaffolds
The design of multilayer structures as vascular scaffolds with respect to the natural layers of the normal vessels and physiological activity of each layer could contribute to the fabrication of similar vascular structure while creating more elasticity, and improving mechanical properties [63]. By using the mixture of natural and synthetic polymers and design changes, it enables us to fabricate desirable structures with density, viscoelastic response, and near-to-natural vessels. Despite advances in the design of the scaffolding, there are still scientific challenges over the design of the ideal scaffolds (Table 2). Here, we point the basic features that are expected from engineered vascular scaffolds.
Biocompatibility
Biocompatibility is touted as one of the most important criteria for evaluating engineering scaffolds. The biocompatible scaffolds lack harmful immunological or pro-inflammatory response after transplantation. The structure and type of transplants must be such that a minimum immunological reaction and recruitment of immune cells happen. The first step in achieving this goal is to apply non-toxic substances. By possessing degradability, transplants will be gently replaced by natural and functional tissues. The use of transplants without these properties contributes to the promotion of immune-mediated reaction and inflammation. In response to these conditions, cell death will happen which in turn exacerbate pathological outcome. Scaffolds should be able to integrate with the host tissue without any harmful immune responses [81, 82]. According to recent data, it was revealed that immune cells notably macrophages could play a critical role after transplantation of scaffolds. Depending on scaffold biocompatibility, macrophages cells could act as double-edged swords and are the frontline of immune defense. During inflammation, macrophages acquire pro-inflammatory phenotype (M1) and after inflammation removal and promotion of healing these cells turn into M2 type. Since scaffold transplantation, phagocytes mainly neutrophils could adhere to the transplant and release numerous hydrolyzing agents and these conditions contribute to initial inflammation. In the next step, macrophages are recalled to the site of transplantation to complete degradation of scaffolds. The non-degradable scaffolds stimulate macrophages to form foreign bodies that are composed of several cells to strengthen degradation capacity. By using appropriate scaffolds with suitable compatibility and degradation rate, macrophages tend to increase polarization to M2 type which could accelerate healing procedure [83].
Porosity
The fabricated scaffolds should be porous with interconnected cavities. The high surface to volume ratio in these constructs could support cell growth and facilitate uniform distribution of cells. The formation and growth of microvessels in the structure become more intense [13, 38, 84]. Therefore, the density of cavities inside constructs and interconnectivity are critical for the proper distribution of nutrients and gases and waste products removal [85, 86]. To achieve proper vessel structure, the porosity and mechanical strength of the scaffolds must be optimized [87].
Pore size
The size of the pore is an integral feature to engineered constructs. In the case, scaffolds with a smaller pore size may affect the cell loading and block the way of cell penetration. The lack of cell penetration not only decreases total cell density per unit volume of transplant but also it prohibits the production of ECM and vascular penetration into a construct [88, 89].
Surface properties
Scaffold surface properties such as surface topography and chemical properties can be effective in cell attachment and proliferation [90, 91]. The adhesion of cells to synthetic surfaces depends on the chemical properties. This index could increases/decreases the number of cells to scaffolds surface which is an inventible character in the engineering of vascular constructs. The existence of topographic properties is cell alignment similar to specific tissue formation and thus controls differentiation rate [92,93,94].
Induction of tissue formation
The induction of tissue formation is a process in which certain cells come together to repair the injured site and differentiate into specific cell lineage [95]. The induction of tissue formation is a phenomenon that can easily be accomplished by using growth factors. Noteworthy, tissue formation can also be induced without growth factors with a special scaffolding design [96].
Biodegradability
Biodegradation is one of the features that should be considered in the scaffolding design [97, 98]. In fact, the temporary filling of tissue defects is one of the main goals by using a distinct scaffold [38, 99]. However, the scaffolds must be decomposed over time with tissue regeneration in order to provide the required conditions for the growth of natural tissue. The decomposition rate of each scaffold must be consistent with the growth rate of the tissue. As a matter of fact, the scaffold should be decomposed when the damage is completely restored. This feature potentiates human medicine to fill the natural tissue defects with biodegradable bio-products while will be removed from the body with tissue healing [48, 100, 101]. It should not be forgotten that scaffolds decomposition will be detrimental and toxic when waste byproducts are at high concentrations [101, 102].
Mechanical properties
The scaffolds used in tissue engineering should be mechanically stable and able to perform biological tasks in the site of the implant. Mechanical stability depends on the biological materials, scaffolding design and cell metabolism [11, 13, 103].
Functionalized scaffolds
This implication stands for a fact that a similar function of constructs is provided, though not a complete function, comparable to the natural counterparts. Modifying the scaffold surface with warfarin, and heparins to prevent blood coagulation is another issue that is considered in the construction of vascular tissue engineering scaffolds. In a better word, engineered vascular grafts must possess anti-thrombogenic property to prevent platelets activation. On the other hand, these modifications such as surface treatment with peptides must lead to a faster and efficient binding of the ECs, but not non-endothelial lineages, to the surface of scaffolds. The successful cell attachment also increases ECs growth and survival which acts as an anti-thrombogenic barrier (Fig. 4).
Electrospun nano-fibers
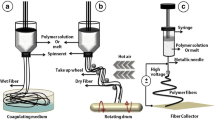
In 1930, electrospinning was first introduced as a simple and economical way to fabricate very thin fibers from a polymeric solution. This technique is touted as one of the most pioneer methods of polymer nanofibers fabrication. Nanostructured scaffolds constructed by electrospinning are prone to mimic a similar condition to ECM. Electrospinning depends on the electrical forces to generate nanofibers. In this technique, nanofibers are produced from a polymeric solution or that is injected from the syringe to a region with a high electric field. When electrostatic forces overcome the surface tension of a polymeric solution, a Taylor cone is formed, and a narrow jet accelerates rapidly towards the target (collector) which is connected to the ground or with the opposite charge (Fig. 5).
Critical factors required for designing of electrospun scaffolds
During the electrospinning process, the morphology control of synthetic polymers is easier than natural polymers. However, according to various benefits of natural polymers such as biological functions, immobilizing them onto the nanofibrous surface of synthetic polymers can be more useful. Since the phenotype and specific cellular organization are highly dependent on the entity of cell binding surface, the use of functionalized nanofibers will be beneficial [106, 108]. There are many factors and components that affect the shape and structure of electrospun nanofibers such as fiber diameter and uniformity. The structure and characteristics of the polymeric materials are affected by chemical structure, molecular weight and spatial order of the chain. In addition, the polymeric solution properties correlate with soluble concentration, viscosity, solvent type, surface tension, electrical conductivity, solvent volatility, solvent dielectric constant, and soluble temperature. To fabricate the nanofibrous scaffolds some process conditions must be considered as follows; applied voltage, spinning distance, feeding rate, the geometry of capillary tube, and collector shape. Notably, environmental factors could affect the quality of fabricated nanofibrous scaffolds. The control of humidity and environmental pressure are fundamental to scaffold synthesis [109, 110].
The application of electrospinning for engineered vascular grafts
Based on the location and type of activity, blood vessels are different in terms of dimensions, mechanical properties, biochemical properties, cell contents, and protoplasmic elements. Therefore, all these properties should be applied in vascular tissue engineering [111]. As above-mentioned, the replacement of vessels, especially narrow vessels with a diameter of less than 6 mm, has many challenges [29]. The culture of ECs on different electrospun nanofibers has been previously studied (Table 3). Data emphasized the capability of electrospinning method to fabricate fibers capable to be used in a wide range of natural and synthetic polymers supplemented with various growth factors [67]. Except for a few cases, most studies are limited to in vivo studies. Tubular scaffolds are made using a varied range of materials. Up to now, many electrospun fibers have been manufactured using a variety of polymers, such as PLGA, elastin polyethylene oxide, PLLA, gelatin/PCL, PCL/PU, ST-gelatin, type I collagen, poly (ethylene oxide), and SPU [112].
Studies have shown that natural polymers have potential to extremely increase cellular connectivity and penetration rate [39, 113]. In recent decades, wide experiments have been done on vascular tissue engineering by the use of electrospun scaffolds. Weinenberg and Bell were the first to develop a model of blood vessels in vitro. They fabricated a multi-layered structure from collagen that integrated with a Dacron mesh and had a similar structure to an artery with potency to endure the physiological pressure. Electron microscopic imaging revealed the existence of ECs in the luminal surface while α-SMA positive cells were located at the structure wall with the ability to acquire functional phenotype. These authors declared that ECs act as a natural barrier of permeability while produced essential factors such as vWF and prostacyclin [51].
A similar technique was used by Hirai and colleagues with similar results. They developed a tubular vascular tissue composed of collagen and vascular cells as a substitute for venous. First, type I collagen and a cold mixed solution of canine jugular α-SMA positive cells were prepared by splashing into a tubular glass mold and incubation at 37 °C. After 10 days of culturing in the medium, they achieved a dense tubular tissue. Then canine jugular ECs seeded on the lumen surface of the tissue to produce a vascular tissue with a hierarchical structure. These engineered vascular tissues that wrapped in Dacron mesh were implanted in the canine posterior vena cava for up to 24 weeks. They observed that The tissues became far dense and go forward in a time-dependent manner and is completed in about 6 months of implantation. [114, 115].
Data from an experiment conducted by Tillman et al. have been shown that electrospun PCL/collagen scaffolds could tolerate physiologic situation whenever preserve functional behavior and attachment of vessels cells. In endothelialized grafts, the reduction of platelet accumulation and hemorrhage was indicated in a rabbit model of the aortoiliac bypass. In addition, these scaffolds were able to maintain their structural integrity within a month after the implantation and these implants exhibited biomechanical endurance that was comparable to a native artery. They suggested that electrospun scaffolds composed with vascular cells may be a good alternative for vascular grafts and reconstruction. [116]. Along with the development of absorbable materials such as PGA, a lot of researches were done on vascular tissue engineering in the 1980s. The application of absorbable materials allows successful replacement with natural tissue with simultaneous degradation of biomaterials. Niklason and colleagues used biodegradable PGA tubes to cultivate bovine aorta α-SMA positive cells for 8 weeks. They also cultivated bovine ECs on the luminal surface of the scaffold. The fabricated vessel structure showed a better rupture strength compared to the native vessels. Interestingly, these structures showed an appropriate contractile response to drug stimulation [117]. In a study done by Campbell and co-workers, they examined the insertion of a silicon tube into the peritoneal cavity in rabbits and mice models. After 2 weeks, a uniform α-SMA positive cells layer was observed similar to the tunica media. Collagen surrounded the tubes as an adventitia layer with the formation of the mesothelium layer [118]. Hajiali and coworkers produced an electrospun scaffold using gelatin and PGA with different ratios (0, 10, 30 and 50 wt.%). To determine the biocompatibility of these scaffolds, they cultivated human umbilical vein endothelial cells and human umbilical artery smooth muscle cells on scaffolds and examined cell attachment and cellular viability. The results showed using PGA with 10 wt% gelatin led to enhance in the endothelial cells and the scaffold made by PGA with 30 wt% gelatin led to increase of cell adhesion, viability, and penetration in the smooth muscle cell, contrasted with the other blends. Also, by increasing the percentage of gelatin due to the intercalation between gelatin and PGA, the mechanical attributes of the scaffold were improved and the scaffolding structure was promising for use in vessel tissue engineering [119]. In another study by Fengyi et al. using chitosan and poly-caprolactone, a heparinized three-dimensional nanofibrous vascular scaffold was developed to prevent thrombosis. They used VEGF and heparin immobilization to mimic the natural blood vessel microenvironment. The results of platelet adhesion assay and activated partial thromboplastin time confirmed that Anti-thrombogenic properties of these scaffolds increased with their heparinization. Also, the adhesion and attachment of HUVECs on CS/PCL scaffold were enhanced. Therefore, the use of CS/PCL heparinized scaffolds could create a method for the production of artificial blood vessel arrays [120].
Vasculogenic cells
Choosing the optimal cell source is one of the challenges in vascular tissue engineering. The use of autologous has always been the best choice because of the immunological responses removal. However, access to an adequate cell resource is not always available. In this regard, the use of stem cells and differentiation into distinct cell types are proposed methods. In addition to autologous stem cells, these cells could be isolated either from allogeneic or xenogeneic candidates. However, in the case of xenogeneic cells, the possibility of transmission of animal viruses is high and thereby the application of allogeneic cells is more logical.
To instruct specific cell function, manipulation is made through genetic engineering of distinct cell type or the change of ECM composition [29, 121]. It should be noted that prolonged culture period with high passage rate can affect cell phenotype. For example, isolated primary ECs can lose their phenotypes and acquire α-SMA like characteristics. To faint these effects, specific culture time and selection of appropriate culture medium are considered as more effective factors compared to cell type selection [122, 123]. Culturing cells in culture media containing VEGF and bFGF has been shown to improve differentiation and express the endothelial-derived factors such as VE-cadherin and vWF. ECs could be isolated from the human saphenous vein, umbilical vein, aorta and mediastinal fat [117, 124, 125] and bovine aorta, pulmonary arteries [126], canine saphenous vein [126]. Adult MSCs and EPCs are alternative cell sources [128, 129].
Ideal properties of vascular grafts
The preparation of an ideal vascular graft requires such features that exist some of them are essential, others favorable. The synthetic vascular graft should have the following characteristics [130]:
- a.
To be biocompatible
- b.
To possess proper mechanical
- c.
To be resistant to infection and inflammation
- d.
To be non-thrombogenic
- e.
To be cost-effective and be simple to use
- f.
To an “off the shelf product” or be easily stock,
- g.
To be accessible in different features such as diameter, length, etc.
Conclusion
The construction of scaffolds by electrospinning for tissue engineering became a popular method every day. A great deal is the ability to build scaffolds with specific structural and functional features. Scaffolds used in vascular tissue engineering should have a layered structure in addition to biocompatibility and anticoagulant properties. Electrospun scaffolds provide a reliable matrix for cellular attachment and support their proliferation, differentiation, integrity, and phenotype. Using this technique, fibers with enhanced mechanical properties can be prepared with different combinations and different decomposition rates. However, precise control of the fiber diameter uniformity and its morphology has some defects in its functionality as an extracellular matrix. But still, the best fiber diameter and porosity between them to optimize cell function is still not fully determined. Although early studies have clearly confirmed the effectiveness of electrospun scaffolds for vascular tissue engineering, scientific literature showed a limit number of in vivo studies with the use of engineered vascular electrospun scaffolds, yet more clinical studies are needed to confirm this theory.
Availability of data and materials
Not applicable
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- bFGF:
-
Basic fibroblast growth factor
- ECM:
-
Extracellular matrix
- ECs:
-
Endothelial cells
- EPCs:
-
Endothelial progenitor cells
- HUVECs:
-
Human umbilical vein endothelial cells
- MSCs:
-
Mesenchymal stem cells
- PCL/Cs:
-
Polycaprolactone/Chitosan
- PGA:
-
Polyglycolic acid
- PLGA:
-
Polylactic-co-glycolic acid
- PLLA:
-
Poly-l-lactide
- SPU:
-
Segmented polyurethane
- ST-gelatin:
-
Styrenated gelatin
- VEGF:
-
Vascular endothelial growth factor
- vWF :
-
von Willebrand’s factor
- α-SMCs:
-
Alpha-smooth muscle cells
References
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511.
Litwinowicz R, Kapelak B, Sadowski J, Kędziora A, Bartus K. The use of stem cells in ischemic heart disease treatment. Kardiochir Torakochirurgia Pol. 2018;15:196–9.
Sontheimer DL. Peripheral vascular disease: diagnosis and treatment. Am Fam Physician. 2006;73 (11):1971–6.
Tranbaugh RF, Schwann TA, Swistel DG, Dimitrova KR, Al-Shaar L, Hoffman DM, Geller CM, Engoren M, Balaram SK, Puskas JD. Coronary artery bypass graft surgery using the radial artery, right internal thoracic artery, or saphenous vein as the second conduit. Ann Thorac Surg. 2017;104(2):553–9.
Syedain Z, Reimer J, Lahti M, Berry J, Johnson S, Bianco R, Tranquillo RT. Corrigendum: tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun. 2017;8:14297.
Bockeria LA, Svanidze O, Kim A, Shatalov K, Makarenko V, Cox M, Carrel T. Total cavopulmonary connection with a new bioabsorbable vascular graft: first clinical experience. J Thorac Cardiovasc Surg. 2017;153(6):1542–50.
Sugi MD, Albadawi H, Knuttinen G, Naidu SG, Mathur AK, Moss AA, Oklu R. Transplant artery thrombosis and outcomes. Cardiovasc Diagn Ther. 2017;3:S219–27.
Awad NK, Niu H, Ali U, Morsi YS, Lin T. Electrospun fibrous scaffolds for small-diameter blood vessels: a review. Membranes. 2018;8(1):15.
Ratcliffe A. Tissue engineering of vascular grafts. Matrix Biol. 2000;19(4):353–7.
Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, Fiorillo A, Avila H, Wystrychowski W, Zagalski K. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30(8):1542–50.
Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354:S32–4.
Xu C, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10(7–8):1160–8.
Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–21.
Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11(1–2):101–9.
Burton AC. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev. 1954;34(4):619–42.
Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19(10):2003–12.
Townsley MI. Structure and composition of pulmonary arteries, capillaries, and veins. Compr Physiol. 2011;2(1):675–709.
Pugsley M, Tabrizchi R. The vascular system: an overview of structure and function. J Pharmacol Toxicol Methods. 2000;44(2):333–40.
L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361.
van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol. 1997;17(4):665–71.
Chen C-W, Corselli M, Péault B, Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439.
Bergmeister H, Strobl M, Grasl C, Liska R, Schima HJES. Tissue engineering of vascular grafts. Eur Surg. 2013;45(4):187–93.
Teebken O, Bader A, Steinhoff G, Haverich A. Tissue engineering of vascular grafts: human cell seeding of decellularised porcine matrix. Eur J Vasc Endovasc Surg. 2000;19(4):381–6.
Pappenheimer JR. Passage of molecules through capillary walls. Physiol Rev. 1953;33(3):387–423.
Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–51.
Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Palo Alto: Annual Reviews; 1998.
Kraehenbuhl J-P, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16(1):301–32.
Cole MA, Quan T, Voorhees JJ, Fisher GJ. Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J Cell Commun Signal. 2018;12:35–43.
Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295(5557):1009–14.
Zhan W, Marre D, Mitchell GM, Morrison WA, Lim SY. Tissue engineering by intrinsic vascularization in an In Vivo tissue engineering chamber. J Vis Exp. 2016;(111):e54099.
Carrel A, Burrows MT. Cultivation of tissues in vitro and its technique. J Exp Med. 1911;13(3):387.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773.
Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. 2012;4(160):160rv112.
Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–3.
Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12(1):697–715.
Hubbell JA. Biomaterials in tissue engineering. Bio/Technology. 1995;13(6):565.
Williams D. The Williams dictionary of biomaterials. Liverpool: Liverpool University Press; 1999. p. 42.
O’brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95.
Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24(24):4353–64.
Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regen Med. 2010;5(1):107–20.
Jaboulay M, Briau E. Recherches expérimentales sur la suture et la greffe artérielles. Lyon Méd. 1896;81:97–9.
Konner K. History of vascular access for haemodialysis. Nephrol Dial Transplant. 2005;20(12):2629–35.
Nosé Y. Dr. Michael E. DeBakey and his contributions in the field of artificial organs. September 7, 1908–July 11, 2008. Artif Organs. 2008;32(9):661–6.
Stewart SF, Lyman DJ. Effects of a vascular graft/natural artery compliance mismatch on pulsatile flow. J Biomech. 1992;25(3):297–310.
Rocco KA, Maxfield MW, Best CA, Dean EW, Breuer CK. In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng B Rev. 2014;20(6):628–40.
Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8–9):762–98.
Sieminski AL, Gooch KJ. Biomaterial–microvasculature interactions. Biomaterials. 2000;21(22):2233–41.
Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59(4–5):207–33.
Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32(3):477–86.
L'heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56.
Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400.
Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen–fibrin mixtures. Biomaterials. 2004;25(17):3699–706.
Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):199–215.
Kogan G, Šoltés L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29(1):17–25.
Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–51.
Schumann DA, Wippermann J, Klemm DO, Kramer F, Koth D, Kosmehl H, Wahlers T, Salehi-Gelani S. Artificial vascular implants from bacterial cellulose: preliminary results of small arterial substitutes. Cellulose. 2009;16(5):877–85.
Bäckdahl H, Helenius G, Bodin A, Nannmark U, Johansson BR, Risberg B, Gatenholm P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials. 2006;27(9):2141–9.
Zhou J, Cao C, Ma X, Lin J. Electrospinning of silk fibroin and collagen for vascular tissue engineering. Int J Biol Macromol. 2010;47(4):514–9.
Kasoju N, Bora U. Silk fibroin in tissue engineering. Adv Healthc Mater. 2012;1(4):393–412.
Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47(1):74–80.
Lantz GC, Badylak SF, Hiles MC, Coffey AC, Geddes LA, Kokini K, Sandusky GE, Morff RJ. Small intestinal submucosa as a vascular graft: a review. J Investig Surg. 1993;6(3):297–310.
Lee KH, Shin SJ, Park Y, Lee SH. Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications. Small. 2009;5(11):1264–8.
Yang S, Leong K-F, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7(6):679–89.
Tillman BW, Yazdani SK, Lee SJ, Geary RL, Atala A, Yoo JJ. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials. 2009;30(4):583–8.
Chlupáč J, Filova E, Bačáková L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res. 2009;58(Suppl 2):S119–39.
Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014;10(1):11–25.
Ku SH, Park CB. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials. 2010;31(36):9431–7.
Alobaid N, Salacinski H, Sales K, Ramesh B, Kannan R, Hamilton G, Seifalian AM. ☆ Nanocomposite containing bioactive peptides promote endothelialisation by circulating progenitor cells: an in vitro evaluation. Eur J Vasc Endovasc Surg. 2006;32(1):76–83.
Kim B-S, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16(5):224–30.
Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129(6):1330–8.
Yamanaka H, Yamaoka T, Mahara A, Morimoto N, Suzuki S. Tissueengineered submillimeter-diameter vascular grafts for free flap survival in rat model. Biomaterials. 2008;179:156–63.
Hoerstrup SP, Kadner A, Breymann C, Maurus CF, Guenter CI, Sodian R, Visjager JF, Zund G, Turina MI. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann Thorac Surg. 2002;74(1):46–52.
Watanabe M, Shin'oka T, Tohyama S, Hibino N, Konuma T, Matsumura G, Kosaka Y, Ishida T, Imai Y, Yamakawa M. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7(4):429–39.
Perry TE, Kaushal S, Sutherland FW, Guleserian KJ, Bischoff J, Sacks M, Mayer JE. Bone marrow as a cell source for tissue engineering heart valves. Ann Thorac Surg. 2003;75(3):761–7.
Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg. 1999;68(6):2298–304.
Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5(8):2145–54.
Lumkwana D, Botha A, Samodien E, Hanser S, Lopes J. Laminin, laminin-entactin and extracellular matrix are equally appropriate adhesive substrates for isolated adult rat cardiomyocyte culture and experimentation. Cell Adh Migr. 2018;12:503–11.
Rose JC, Gehlen DB, Haraszti T, Köhler J, Licht CJ, De Laporte L. Biofunctionalized aligned microgels provide 3D cell guidance to mimic complex tissue matrices. Biomaterials. 2018;163:128–41.
Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater. 2018;3:159–73.
Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518.
Kim B-S, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16(5):224–30.
Mikos AG, Temenoff JS. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron J Biotechnol. 2000;3(2):23–4.
Wissing TB, Bonito V, van Haaften EE, van Doeselaar M, Brugmans MMCP, Janssen HM, Bouten CVC, Smits AIPM. Macrophage-driven biomaterial degradation depends on scaffold microarchitecture. Front Bioeng Biotechnol. 2019;7:87.
Lu L, Mikos AG. The importance of new processing techniques in tissue engineering. MRS Bull. 1996;21(11):28–32.
Barbetta A, Dentini M, De Vecchis MS, Filippini P, Formisano G, Caiazza S. Scaffolds based on biopolymeric foams. Adv Funct Mater. 2005;15(1):118–24.
da Silva J, Lautenschläger F, Sivaniah E, Guck JR. The cavity-to-cavity migration of leukaemic cells through 3D honey-combed hydrogels with adjustable internal dimension and stiffness. Biomaterials. 2010;31(8):2201–8.
Hollister SJ, Maddox R, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23(20):4095–103.
O’Brien FJ, Harley B, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26(4):433–41.
Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31(3):461–6.
Xie J, MacEwan MR, Li X, Sakiyama-Elbert SE, Xia Y. Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano. 2009;3(5):1151–9.
Grover CN, Gwynne JH, Pugh N, Hamaia S, Farndale RW, Best SM, Cameron RE. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012;8(8):3080–90.
Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro-and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48(30):5406–15.
Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci. 2006;103(8):2480–7.
Saghati S, Akbarzadeh A, Del Bakhshayesh A, Sheervalilou R, Mostafavi E. Electrospinning and 3D printing: prospects for market opportunity. In: Electrospinning. In; 2018. p. 136–55.
Lutolf M, Hubbell J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47.
Patel S, Caldwell JM, Doty SB, Levine WN, Rodeo S, Soslowsky LJ, Thomopoulos S, Lu HH. Integrating soft and hard tissues via interface tissue engineering. J Orthop Res. 2018;36(4):1069–77.
Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55(2):141–50.
Nam YS, Park TG. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res. 1999;47(1):8–17.
Ma PX. Scaffolds for tissue fabrication. Mater Today. 2004;7(5):30–40.
Sionkowska A, Kaczmarek B, Lewandowska K, Grabska S, Pokrywczyńska M, Kloskowski T, Drewa T. 3D composites based on the blends of chitosan and collagen with the addition of hyaluronic acid. Int J Biol Macromol. 2016;89:442–8.
Cheung H-Y, Lau K-T, Lu T-P, Hui D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos Part B Eng. 2007;38(3):291–300.
Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol. 2010;47(1):1–4.
Hutmacher D, Goh J, Teoh SH. An introduction to biodegradable materials for tissue engineering applications. Ann Acad Med Singapore. 2001;30(2):183–91.
Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28(22):3338–48.
Rezwan K, Chen Q, Blaker J, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–31.
Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518.
Chen G, Ushida T, Tateishi T. Scaffold design for tissue engineering. Macromol Biosci. 2002;2(2):67–77.
Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y). 1994;12(7):689.
Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–47.
Schiffman JD, Schauer CL. A review: electrospinning of biopolymer nanofibers and their applications. Polym Rev. 2008;48(2):317–52.
Chaudhuri S, Chatterjee S, Katz N, Nelson M, Goldbaum M. Detection of blood vessels in retinal images using two-dimensional matched filters. IEEE Trans Med Imaging. 1989;8(3):263–9.
Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–53.
Li D, Xia Y. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004;4(5):933–8.
Hirai J, Kanda K, Oka T, Matsuda T. Highly oriented, tubular hybrid vascular tissue for a low pressure circulatory system. ASAIO J. 1994;40(3):M383–8.
Hirai J, Matsuda T. Venous reconstruction using hybrid vascular tissue composed of vascular cells and collagen: tissue regeneration process. Cell Transplant. 1996;5(1):93–105.
Tillman BW, Yazdani SK, Lee SJ, Geary RL, Atala A, Yoo JJ. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials. 2009;30(4):583–8.
Niklason L, Gao J, Abbott W, Hirschi K, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284(5413):489–93.
Campbell JH, Efendy JL, Campbell GR. Novel vascular graft grown within recipient’s own peritoneal cavity. Circ Res. 1999;85(12):1173–8.
Hajiali H, Shahgasempour S, Naimi-Jamal MR, Peirovi H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int J Nanomedicine. 2011;6:2133.
Du F, Wang H, Zhao W, Li D, Kong D, Yang J, Zhang Y. Gradient nanofibrous chitosan/poly ɛ-caprolactone scaffolds as extracellular microenvironments for vascular tissue engineering. Biomaterials. 2012;33(3):762–70.
Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7.
Campbell GR, Campbell JH. Development of tissue engineered vascular grafts. Curr Pharm Biotechnol. 2007;8(1):43–50.
Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90(11):1189–96.
Tiwari A, DiSalvo C, Walesby R, Hamilton G, Seifalian AM. Mediastinal fat: a source of cells for tissue engineering of coronary artery bypass grafts. Microvasc Res. 2003;65(1):61–4.
Matsuda T, He H. Newly designed compliant hierarchic hybrid vascular grafts wrapped with a microprocessed elastomeric film—I: Fabrication procedure and compliance matching. Cell Transplant. 2002;11(1):67–74.
Jones PA. Construction of an artificial blood vessel wall from cultured endothelial and smooth muscle cells. Proc Natl Acad Sci U S A. 1979;76(4):1882–6.
He H, Matsuda T. Newly designed compliant hierarchic hybrid vascular graft wrapped with microprocessed elastomeric film—II: morphogenesis and compliance change upon implantation. Cell Transplant. 2002;11(1):75–87.
Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9(1):127–36.
Cho S-W, Lim SH, Kim I-K, Hong YS, Kim S-S, Yoo KJ, Park H-Y, Jang Y, Chang BC, Choi CY. Small-diameter blood vessels engineered with bone marrow–derived cells. Ann Surg. 2005;241(3):506.
Conte MS. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998;12(1):43–5.
Acknowledgments
All authors wish to thank the personnel of the Faculty of Advanced Medical Sciences for guidance and help.
Funding
This review article is supported by a grant (IR.TBZMED.VCR.REC.1397.264) from Tabriz University of Medical Sciences. Is supported by a grant from Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
SFK, RS, AA. Data collection and manuscript writing; SD, and RR: Equal conceptualization and manuscript edition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Karkan, S.F., Davaran, S., Rahbarghazi, R. et al. Electrospun nanofibers for the fabrication of engineered vascular grafts. J Biol Eng 13, 83 (2019). https://doi.org/10.1186/s13036-019-0199-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13036-019-0199-7