Abstract
Background
In patients with cardiovascular diseases, it is reported that the triglyceride-glucose index (TGI) potentially indicates prognosis. However, the results are controversial. Moreover, whether age has an impact on the predictive value of TGI remains unclear.
Methods
Participants with cardiovascular diseases were enrolled using the China Health and Retirement Longitudinal Study (CHARLS) registry. TGI was calculated as ln (triglyceride×glucose/2). The survival status was recorded every 2 years in the follow-up waves. Multivariate regression analysis was carried out to determine the relationship between TGI levels and long-term all-cause mortality in patients grouped by different age. Patients younger than 65 years old were regarded as middle-aged group. Otherwise, they were classified as old group.
Results
In total, 2923 patients with cardiovascular diseases and baseline blood test results were included. After 7 years of follow-up, 242 (8.91%) patients died. Cox regression analysis revealed that higher TGI levels were associated with a higher risk of long-term all-cause mortality in middle-aged participants (hazard ratio [HR], 3.64; 95% confidence interval [CI] 1.44–9.22, P = 0.006) but not in old participants (HR 1.20, 95% CI 0.62–2.32, P = 0.594, P for interaction = 0.017), after adjusting physical activity and other factors. Kaplan–Meier estimate analysis and restricted cubic spline curves showed similar results.
Conclusion
TGI was a promising marker for predicting all-cause mortality in middle-aged patients after cardiovascular diseases. Patients younger than 65 years old who have a higher level of TGI may develop a higher risk of all-cause mortality, and they are encouraged to control vascular risk factors and take more physical activity to improve their prognosis. Additionally, whether intervention in regulating TGI levels is beneficial for the prognosis of these patients needs further investigation.
Similar content being viewed by others
Background
It is well known that cardiovascular diseases are one of the most important diseases [1]. Patients with cardiovascular diseases have restricted heart function and poor quality of life, causing a serious disease burden on their families and society [2]. Exploring potentially remediable risk factors is of major significance to improve the prognosis after cardiovascular diseases [3].
Many novel risk factors, such as low insulin sensitivity, play a significant role in the prognosis of cardiovascular diseases [4,5,6,7]. Insulin resistance was related to all-cause mortality in general healthy person or patients with cardiovascular risk factors [8]. Currently, common methods used to detect insulin resistance include glucose tolerance testing and the homeostasis model assessment of insulin resistance (HOMA-IR) [9]. However, these tests are complicated and have not been commonly used in clinical practice [10,11,12].
Recently, the triglyceride-glucose index (TGI) has been developed as an indicator of low insulin sensitivity [13,14,15] and was correlated with other indicators such as HOMA-IR [16]. It was suggested that participants with higher TGI levels were predisposed to diabetes [17], hypertension [18], obesity [19], atherosclerosis [20], and cardiovascular diseases [21], all of which are significantly related to a higher risk of mortality in patients with cardiovascular diseases [22]. Recently, several studies have revealed that higher TGI levels are independently related to poorer prognosis after cardiovascular diseases [23, 24]. However, some other studies reported different results and indicated that TGI was not significantly associated with the risk of unfavorable prognosis in patients with cardiovascular diseases [25, 26]. This inconsistency may be due to the different characteristics of the participants.
TGI is associated with many factors, such as age and fasting state. It was reported that age was inversely correlated with TGI, and younger patients had higher levels of TGI [25, 27,28,29,30]. Furthermore, an increased volume of physical activity reduces the level of insulin resistance [31]. Given that younger patients typically exhibit a higher volume of physical activity than older patients [32], a complex relationship may exist between age, physical activity, and TGI. To determine whether age could modify the association between TGI and mortality and the value of physical activity in the potential interaction between age and TGI after cardiovascular diseases, a detailed analyses of data using the China Health and Retirement Registry (CHARLS) were performed.
Methods
Data collection
The CHARLS has been an international survey for middle-aged and old Chinese, and the data used are available by visiting the official website [33]. The initial study was launched in 2011 (wave 1), and the individuals were followed up after the initial study to explore the determinants and consequences of aging. The follow-up wave 2, wave 3, and wave 4 were released in 2013, 2015, and 2018, respectively. In total, 25,586 individuals were interviewed after combining the initial samples and refreshment samples. CHARLS collected data including past-history, health and lifestyle status, present illness, work and income, and social networks from the collected samples of 28 Chinese provinces covering 150 districts and 450 communities. The study provided detailed information for medical, social, political, and economic research. Many studies have been published based on this nationally representative database [33]. All data were obtained by interview and examination.
The inclusion criteria were 1) individuals with complete baseline data on age, gender, and history of diseases; 2) individuals with a history of cardiovascular diseases; and 3) individuals with data on plasma levels of glucose and triglycerides. Correspondingly, individuals without plasma glucose and triglycerides results were excluded. Self-reported baseline data, including demographic characteristics, chronic diseases, and lifestyle factors, were collected. Education was classified into 3 types. Specialists performed physical examination to collect body measurements [33]. Blood pressures were measured by a sphygmomanometer (Omron, Japan) three times for every participant [33]. Body mass index (BMI) was also obtained by measuring height and weight.
Cardiovascular diseases were defined as coronary artery diseases, valve heart diseases, heart failure, arrhythmia, other heart diseases, and stroke [33]. Hypertension, diabetes, dyslipidemia, and stroke were defined as past-history, or elevated blood pressure, glucose, or lipids according to diagnostic criteria, or the use of corresponding drugs [34,35,36]. Other chronic diseases were determined by self-reported history.
Participants responded to questions surveying the volume of physical activity. Vigorous activity includes activities such as dancing, swimming, and strenuous running. Moderate physical activity includes light housework and brisk running with harder breath than usual conditions, and mild physical activity includes walking with a normal pace and routine work with light intensity. The duration and frequency were applied to calculate the volume. For vigorous physical activity, the volume was classified as no activity, less than 75 min every week, greater than 75 and less than 300 min every week, and greater than 300 min every week [37]. Moderate and mild physical activity was classified into no activity, less than 150 min every week, greater than 150 and less than 300 min every week, and greater than 300 min every week, according to World Health Organization guideline [37].
A hematological examination was conducted by a special laboratory. Medical staff collected venous blood from participants. Blood routine tests were measured by automated analyzers within 141 min of collection. The plasma samples were put in cryovials at below 20°C, transported to Beijing, and then stored at below 80°C until assayed. Blood glucose and lipids were assayed by enzymatic colorimetric test methods [38, 39]. The detection limit of blood glucose was 2-450 mg/dl. The analytical range of triglycerides was 4-1000 mg/dl. TGI was calculated using the formula based on previous study [40]. The blood test results in wave 1 (in 2011) were regarded as baseline data. In wave 3 (in 2015), randomly collected blood sample data were regarded as follow-up data.
Outcome assessment
The follow-up interview waves provided information on participants’ survival status (dead or alive). For participants who died before wave 2 (in 2013), the exact time and the cause of death were recorded. The survival time of these participants was evaluated by the time span between the time of recruitment in CHARLS and the exact death time. For participants who died after wave 2, only the survival status was recorded. If the time of death was unavailable, the survival time of these participants was calculated by the median of the time span between baseline time and the time of follow-up wave with death information. For participants who were alive through all waves, the survival time was the time span between the baseline wave and the last wave. Cardiovascular and non-cardiovascular mortality were defined as death due to or not due to cardiovascular diseases, respectively; non-cardiovascular mortality included death due to cancer, lung diseases, and other non-cardiovascular diseases.
Statistical analysis
Patients were first divided into different groups with ages younger and older than 65 years (middle-aged patients and old patients). Then, the enrolled participants were further classified into the low TGI group and the high TGI group by the Receiver operating characteristic (ROC) curve. The demographic data, vascular factors, and blood test results were compared among these groups. Continuous data were displayed as the mean as well as standard deviation (SD) for normally distributed data. Variables, such as BMI, triglycerides, and C-reactive protein (CRP), were reported as the median. The difference between 2 TGI groups was conducted by a t-test or the Mann‒Whitney U test [21]. Categorical data are reported as numbers and proportions. Their differences were assessed by the chi-square test [21]. The effect of fasting state and cardiovascular-related drugs on the levels of triglycerides, glucose, and TGI was also explored. The spearmen correlation coefficient was also calculated for TGI and age as well as physical activity, and the distribution of physical activity were compared.
Cox regression was carried out to explore factors for all-cause mortality in middle-aged patients and old patients. Hazard ratios (HRs) as well as 95% confidence intervals (CIs) were obtained. First, univariate analysis was performed; and then multivariate analysis was performed with 2 models using potential factors according to univariate analysis (P < 0.05). In model 1, potential confounding factors were entered into multivariate analysis. In model 2, physical activity was further adjusted. Likelihood ratio tests were used to detect the interaction between different age groups.
To illustrate the different associations between TGI and mortality in middle-aged patients and old patients, Kaplan–Meier estimate curves and restricted cubic spline curves were generated. The differences in mortality rates were tested using the log-rank test. Then, association between fully adjusted HR for TGI levels predicting the risk of mortality in middle-aged and old groups was displayed by a restricted cubic spline curve, with four knots of TGI distribution. The median percentile of the TGI distribution was applied as a reference level [41].
To compare the prognostic value between glucose, triglycerides, and TGI, area under the curve of ROC, integrated discrimination improvement score, and net reclassification improvement score of adding glucose, triglycerides, and TGI into the original model (model 2) were calculated. To further identify whether the value of TGI on mortality in different age groups was modified by other factors, subgroup analyses including sex, diabetes, hypertension, BMI, waist circumference, physical activity, and fasting state were carried out. To explore the potential interaction between these factors and TGI levels, the P for interaction in each subgroup analysis was also calculated. For the sensitivity analysis, different cutoff points for age (55, 60, 65, and 70 years old) were used to recalculate HR and 95% CI. The statistical analysis was conducted by Stata 15.0 and GraphPad 8.0.
Results
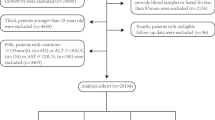
Of the 11636 participants only with plasma glucose data and 11656 participants only with plasma triglycerides data, 11636 participants had data for both glucose and triglycerides. Among them, 2923 patients with cardiovascular diseases were followed up in the subsequent waves. The average age was 60.27 ± 9.38 years, and 1151 (39.38%) patients were males. Among the 2923 patients, 2065 (70.65%) middle-aged patients were 55.59 ± 6.20 years. Furthermore, 858 (29.35%) old patients were 71.82 ± 4.72 years. The TGI in middle-aged patients (8.80 ± 0.71) was higher than that in old patients (8.73 ± 0.66, P = 0.007). The mean TGI was 8.78 (SD: 0.70), and the optimal cutoff value for TGI level was 8.61.
Patients without fasting had higher levels of TGI than patients with fasting (Supplementary Table 1). Patients treated with drugs for hypertension, diabetes, and dyslipidemia had higher levels of baseline TGI (in 2011) and follow-up TGI (in 2015) than those who did not (Supplementary Table 2). The TGI ranged from 4.96 to 8.61 in 1299 patients, in the low TGI group and ranged from 8.61 to 12.96 in 1624 patients, in the high TGI group. Finally, patients were divided into 4 groups.
Table 1 shows the detailed baseline data. In old patients, patients with high TGI levels (average age: 71.50) were younger than those with low TGI levels (average age: 72.15, P = 0.045). Additionally, in middle-aged group, the high TGI group had more male patients; in old group, the high TGI group had fewer male patients (P < 0.001). In both middle-aged and old groups, whose TGI levels were higher had increased levels of BMI, glucose, triglycerides, white blood cells, platelets, glycated hemoglobin, low-density lipoprotein (LDL), total cholesterol levels, and decreased levels of high-density lipoprotein (HDL). The proportions of patients with diabetes, hypertension, and dyslipidemia were also higher in the high TGI group. However, the proportions of patients with drinking and smoking habits were lower in the high TGI group. In middle-aged patients, the proportion of patients participating in vigorous physical activity was lower in the high TGI groups (P = 0.049).
Furthermore, TGI was inversely correlated with age and moderate physical activity. Patients with old age had a lower volume of vigorous, moderate, and mild physical activity. Moreover, patients with older age and higher TGI levels had an increased rate of physical inactivity than patients with younger age and lower TGI levels (Supplementary Figs. 1-3).
After 7 years, 207 (7.08%) patients did not have information on follow-up data, and 242 (8.28%) patients died. Furthermore, in middle-aged patients, the mortality increased from 3.01% of the low TGI group to 5.09% of the high TGI group (P = 0.024). However, in old patients, the rate of death did not significantly differ between patients with low TGI levels and those with high TGI levels (19.73% vs. 21.17%, P = 0.619). Supplementary Fig. 4 shows that in middle-aged patients, cardiovascular mortality (31.87%) tended to be the dominant cause of death; while in old patients, non-cardiovascular mortality (30.49%) was more prominent.
In middle-aged patients, univariate analysis suggested that TGI was positive associated with mortality (Table 2); moreover, higher TGI levels were independently related to an increased risk of mortality (HR 2.48, 95% CI 1.28–4.85, P = 0.008) after adjusting for potential confounding factors. However, in old patients, TGI was not significantly related to the risk of all-cause mortality in univariate or multivariate analysis. Importantly, a significant interaction between TGI and age was seen (P = 0.024). Furthermore, after further adjusting for vigorous physical activity, middle-aged patients with higher TGI levels had a 2.64 times increased risk of mortality than middle-aged group with lower TGI levels (HR 3.64, 95% CI 1.44–9.22, P = 0.006). Nevertheless, for old patients, further adjustment only slightly changed the nonsignificant predictive value (P = 0.594; P for interaction between middle-aged and old groups: 0.017).
Furthermore, compare with glucose and triglycerides, TGI showed a better predictive value by using the ROC analysis and 2 discriminative scores (Supplementary Table 3). Supplementary Table 4 shows relative values of HRs were higher in the middle-aged patients and significant interactions were observed in most groups. In middle-aged groups divided by 65 and 70 years old, the association of TGI predicting all-cause mortality was significant.
Consistently, similar results were observed in Kaplan‒Meier estimate analysis, which illustrated the estimates of mortality among middle-aged patients (Fig. 1a) and old patients (Fig. 1b). In middle-aged patients, compared with the low TGI group, the risk of mortality was significantly higher in the high TGI group (Fig. 1a, P for log-rank test = 0.024). However, in old patients, the P for log-rank test was 0.573, suggesting insignificant results (Fig. 1b).
To identify the linear relationship in middle-aged and old patients, a spline curve was generated to delineate the change in mortality. Figure 2a shows that in middle-aged patients, HRs increased along with TGI levels, suggesting an elevated risk of mortality with increases in the magnitude of TGI levels. However, in old patients, no dose-response relationship was observed (Fig. 2b).
Adjusted dose-response association between triglyceride glucose index levels and the risk of all-cause mortality in middle-aged patients (a) and old patients (b). TGI was coded using a restricted cubic spline function with four knots located at the 25th, 50th, 75th, and 95th percentiles of the distribution of TGI. Reference TGI value is 8.74 in (a) and 8.65 in (b). Y-axis represents the adjusted hazard ratios of all-cause mortality risk when comparing patients with any value of TGI with patients with the reference value of TGI. Dashed lines are 95% confidence intervals. Adjusted factors included age, sex, systolic blood pressure, BMI, waist, diabetes, hypertension, dyslipidemia, drinking, smoking, white blood cells, platelet, creatinine, hematocrit, hemoglobin, glycated hemoglobin, HDL, LDL, total cholesterol, and vigorous physical activity. Abbreviation: TGI, triglyceride glucose index, BMI, body mass index, HDL, high-density lipoprotein, LDL, low-density lipoprotein
To explore whether sex, diabetes, hypertension, BMI, waist circumference, and vigorous physical activity interact with TGI to predict all-cause mortality, stratified analyses were performed using these factors, and the stratified factor was not adjusted in each analysis. No additional variables that modified the association between TGI levels and mortality in middle-aged and old patients were identified (Table 3). In middle-aged patients, TGI was a predictor of mortality in male patients, patients without diabetes, patients with larger BMI, patients with larger waist circumference, patients without vigorous physical activity, and patients with fasting TGI. Nevertheless, in old patients, TGI was significantly related to mortality only in patients with BMI greater than 24 kg/m2.
Discussion
This study suggested higher TGI levels were significantly related to a higher risk of all-cause mortality in middle-aged patients. The association was only significant in middle-aged patients, but not in old patients. A significant interaction was observed between TGI and age for predicting the risk of all-cause mortality.
Additionally, considering that physical activity was one of the main influencing factors of insulin resistance, the relationship among age, physical activity, and TGI was also explored. TGI levels were associated with poor prognosis regardless of whether physical activity was adjusted. In the subgroup analysis, the association was significant in middle-aged patients without vigorous physical activity. These results suggested that patients without adequate physical activity may have a severe degree of insulin resistance, which may increase the risk of mortality. Therefore, middle-aged patients should perform more physical activity to potentially reduce the risk of poor prognosis after cardiovascular diseases. Further studies should explore whether participating in more physical activity could decrease TGI levels and thus improve prognosis in middle-aged patients with cardiovascular diseases.
Consistent with the present study, Ma et al. found participates with elevated TGI levels had a higher rate of major adverse cardiovascular events in middle-aged patients but not in old patients [27]. However, subgroup analysis for all-cause mortality stratified by age was not performed [27]. Interestingly, although Yang et al. revealed that TGI levels may be associated with unfavorable prognosis, they found different results in patients of different ages grouped with 65 years old [25]. TGI tended to be a marker of good prognosis in younger patients, and an indicator of poor prognosis in older patients [25].
In contrast, the present study indicated that TGI was a predictor of prognosis exclusively in middle-aged patients, with a significant interaction between age and TGI. This differs from those of Yang et al. given the following possible explanation. First, the patients included were different. All kinds of cardiovascular diseases were included in this study. Nevertheless, Yang et al. only selected patients without diabetes who received PCI. Patients who undergo acute surgery are susceptible to hyperglycemia, which may have an impact on the true effect of TGI on outcomes [42]. Second, the outcome measurements were different. Yang et al. included 2-year major adverse cardiovascular events as outcome indicators. The rate of all-cause mortality in their study was relatively low (1%). Thus, it was impossible to directly compare the present study with their studies. Third, age has an impact on the degree of insulin resistance, which may partially explain the complex role of age in the association between TGI and prognosis. It has been reported that younger patients are more susceptible to insulin resistance [25], and have higher TGI levels [25, 27,28,29,30]. Younger participants with high levels of TGI may have an elevated risk of insulin resistance-related diseases such as diabetes and obesity, which may increase the risk of mortality [43]. Thus, TGI was a significant marker to represent the risk of mortality in relatively young participants.
Of note, it was found no significant results for TGI predicting mortality in old patients, and the reason was unclear. In this study, old patients had more proportion of non-cardiovascular mortality. Previously, TGI was a marker of cardiovascular diseases and cardiovascular mortality [44]. Therefore, the weak relationship between TGI and non-cardiovascular mortality may partly explain the unrelatedness in old patients. It is worth investigating whether the lower distinguishing ability of TGI in older patients is due to the different predictive abilities of TGI for cardiovascular and non-cardiovascular mortality.
Previously, studies have suggested that higher TGI levels were related to an elevated risk of poor outcomes in participants with coronary artery diseases [45, 46], patients with nonobstructive coronary arteries [47], patients with coronary heart diseases [27, 44]. These studies suggested the potential role of TGI levels in predicting clinical outcomes after cardiovascular diseases.
However, other studies proposed that TGI levels were not independently related to outcomes [25, 26, 48]. Vega et al. suggested that TGI levels were not related to mortality in the general male population [48]. Apparently, the results exhibited gender selection bias. Another study included 1340 myocardial infarction patients without diabetes, and concluded that TGI was not an indicator of mortality [26]. Furthermore, Wang et al. suggested that TGI was not correlated with mortality but was associated with major adverse cardiovascular events [44]. The low rate of mortality in their study made it difficult to identify the association. Additionally, Zhao et al. indicated that Kaplan–Meier curves of poor prognosis did not significantly differ between groups with different TGI levels [12]. Numerous differences in patient selection, outcome definition, and study design were noted in previous studies, all of which may contribute to the inconsistency of these results. Therefore, this calls for more studies to explore the potential modifiers.
Several possible mechanisms may explain the positive association [9]. TGI reflects hyperglycemia and hyperlipidemia. Higher blood glucose could induce oxidative stress, causing endothelial dysfunction [49,50,51]. Elevated plasma lipids promote the formation of atherosclerotic plaques [52]. In addition, hyperglycemia and dyslipidemia increase thrombotic events to increase the risk of atherosclerotic plaque rupture [20], cardiovascular event recurrence [53,54,55] and mortality [53, 56]. The aforementioned mechanisms might partly give the reason for the potential relationship between TGI and mortality risk in patients with cardiovascular diseases.
Previously, many research have revealed the association between low insulin sensitivity and poor outcome [57, 58]. Insulin resistance is related to energy dysmetabolism, oxidative stress, vascular remodeling, and poor endothelial function, all of which result in higher myocardial oxygen consumption, lower myocardial compensatory capacity, and reduced collateral formation [59,60,61,62,63,64]. These factors lead to poorer myocardial reperfusion and coronary microcirculation, larger infarct size, and more adverse cardiovascular events [65].
Moreover, traditional cardiovascular risk factors may be the bridge between TGI and mortality. TGI is also associated with BMI, waist circumference, glycated hemoglobin, LDL, and total cholesterol levels, indicating that patients with higher TGI have more cardiovascular risk factors, which may lead to poor prognosis of patients with cardiovascular diseases. Finally, TGI may increase the severity of cardiovascular diseases to increase the risk of death. Higher TGI levels are associated with more serious cardiovascular diseases, coronary calcification and stenosis, atheroma plaque formation rupture, micro- and macroangiopathies, and cardiac autonomic neuropathy [66,67,68], and all of these conditions increase the incidence of adverse cardiovascular events. Participants with higher TGI levels had an increased risk of acute vascular events [21], thus increasing the risk of mortality.
Of note, TGI is a simple, low-cost, and routinely measured surrogate indicator that reflects the long-term risk of mortality compared with other markers. Thus, TGI may exhibit promise promising to be applied in future clinical applications to help the early selection of middle-aged patients who have a high risk of mortality. In short, TGI may provide critical value for risk stratification in middle-aged patients. Patients with a higher TGI level should be closely monitored to evaluate the risk of mortality. Another important consideration for the application of TGI is the influence of other factors, such as the fasting state and weight. Fasting TGI may have a better predictive value than non-fasting TGI. Although the interaction between TGI and fasting state as well as BMI in the present study was not significant, many studies have explored the predictive value of combining TGI with other markers such as TGI combining with BMI [69]. Therefore, other factors related to TGI should also be considered before the usage of TGI in clincial practice.
Importantly, decreasing the levels of insulin resistance may benefit clinical prognosis of patients with cardiovascular disease [70]. Although previous studies have suggested many strategies to reduce the level of TGI, the role of these strategies in improving prognosis remains unknown. For example, a special diet, such as whole-grain consumption, decreased the level of insulin resistance [71], and high carbohydrate consumption and low lipid consumption reduced the risk of developing high TGI levels [72]. It is unclear whether intervention to lower TGI could improve the prognosis of these patients and whether TGI can serve as an indicator to evaluate the role of therapies on insulin resistance intervention. Additionally, it is unclear the effect of cardiovascular diseases related drugs on the levels of TGI. This post-hoc analysis suggested patients using drugs for hypertension, diabetes, and dyslipidemia had higher TGI levels. However, the inverse cause-effect relationship may exist. Whether therapies, such as statins and antidiabetic drugs, could decrease the levels of TGI to improve the prognosis in patients with high TGI levels remains to be investigated by high-quality clinical randomized control trials [73].
Furthermore, it is worth noting that other biomarkers such as hemoglobin [74] and troponin [75] also show great predictive value for patients with cardiovascular diseases. Previously, researchers have established some predictive models with clinical factors and biomarkers to evaluate the prognosis [76, 77], there need more studies to investigate whether combining TGI with other promising biomarkers could improve the predictive value of these prediticve models.
Strengths and limitations
The present study provides novel information for the potential application of TGI in middle-aged patients. Moreover, It is insipiring to propose that age may be a potential modifier for TGI predicting mortality after cardiovascular diseases.
However, it also has several limitations. First, middle-aged and old adults were included. Therefore, it is unclear whether the significant association between TGI and age also occurred in individuals younger than 45 years old. The mortality in young patients was low, further investigations are needed to figure out the value of TGI in a relatively low-mortality rate population. Additionally, other insulin-resistance markers, such as HOMA-IR, were not compared with TGI in the prediction of mortality. However, considering that biochemical tests are more widely used, TGI may be a more easily obtained and promising routinely used marker for predicting mortality than other markers.
Moreover, the data on the cause of death was unavailable in some patients, so the association between TGI and different cause of mortality were not analyzed. TGI is a marker of cardiovascular diseases and cardiovascular mortality [78], and whether TGI is also related to non-cardiovascular mortality is unclear. Therefore, it is worth investigating the ability of TGI to distinguish different causes of mortality. Lastly, the study included participants with all types of cardiovascular diseases. The datasets had no information on some certain types of cardiovascular diseases influenced by insulin resistance such as arteriosclerotic cardiovascular disease (ASCVD), further studies should be conducted to clarify whether TGI had a better prognostic value for ASCVD than other types of cardiovascular diseases.
Conclusion
TGI was a promising marker for predicting all-cause mortality in middle-aged patients after cardiovascular diseases. Patients younger than 65 years old who have a higher level of TGI may develop a higher risk of all-cause mortality, and they are encouraged to control vascular risk factors and take more physical activity to improve their prognosis. Additionally, whether intervention in regulating TGI levels is beneficial for the prognosis of these patients needs further investigation.
Availability of data and materials
The data are available from http://charls.pku.edu.cn.
Abbreviations
- TGI:
-
Triglyceride-glucose index
- HOMA-Insulin Resistance:
-
The homeostasis model assessment of IR
- CHARLS:
-
The China Health and Retirement Registry
- BMI:
-
Body Mass Index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- ROC:
-
Receiver Operating Characteristic curve
- SD:
-
Standard deviation
- CRP:
-
C-Reactive Protein
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
- PCI:
-
Percutaneous coronary intervention
- ASCVD:
-
Arteriosclerotic cardiovascular disease
References
Sacco RL, Roth GA, Reddy KS, Arnett DK, Bonita R, Gaziano TA, et al. The heart of 25 by 25: achieving the goal of reducing Global and Regional premature deaths from Cardiovascular Diseases and Stroke: a modeling study from the American Heart Association and World Heart Federation. Global Heart. 2016;11(2):251 – 64. https://doi.org/10.1016/j.gheart.2016.04.002.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010.
Rudnicki M, Haas TL. Exploring risk factors at the molecular level. eLife. 2021;10. https://doi.org/10.7554/eLife.68271.
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. https://doi.org/10.1007/s00125-002-1009-0.
Ye Z, Xie E, Jiao S, Gao Y, Li P, Tu Y, et al. Triglyceride glucose index exacerbates the risk of future cardiovascular disease due to diabetes: evidence from the China Health and Retirement Longitudinal Survey (CHARLS). BMC Cardiovasc Disord. 2022;22(1):236. https://doi.org/10.1186/s12872-022-02673-y.
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19(1):155. https://doi.org/10.1186/s12933-020-01121-5.
Li H, Zuo Y, Qian F, Chen S, Tian X, Wang P, et al. Triglyceride-glucose index variability and incident cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):105. https://doi.org/10.1186/s12933-022-01541-5.
Zhang X, Li J, Zheng S, Luo Q, Zhou C, Wang C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: a meta-analysis. Biosci Rep. 2017;37(5). https://doi.org/10.1042/bsr20170947.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. https://doi.org/10.1186/s12933-022-01511-x.
Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1(2):36–47. https://doi.org/10.4239/wjd.v1.i2.36.
Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10(1):2–42. https://doi.org/10.2174/1573399810666140214093600.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. https://doi.org/10.1186/s12933-020-01086-5.
Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. https://doi.org/10.1186/s12933-014-0146-3.
Vasques ACJ, Novaes FS, de Oliveira MdS M, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100. https://doi.org/10.1016/j.diabres.2011.05.030.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. https://doi.org/10.1089/met.2008.0034.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. https://doi.org/10.1210/jc.2010-0288.
Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural chinese cohort study. Cardiovasc Diabetol. 2017;16(1):30. https://doi.org/10.1186/s12933-017-0514-x.
Sánchez-Íñigo L, Navarro-González D, Pastrana-Delgado J, Fernández-Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a spanish cohort. J Hypertens. 2016;34(7):1257-65. https://doi.org/10.1097/hjh.0000000000000941.
Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. 2016;17(6):458 – 65. https://doi.org/10.1111/pedi.12303.
Zhao X, Wang Y, Chen R, Li J, Zhou J, Liu C, et al. Triglyceride glucose index combined with plaque characteristics as a novel biomarker for cardiovascular outcomes after percutaneous coronary intervention in ST-elevated myocardial infarction patients: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2021;20(1):131. https://doi.org/10.1186/s12933-021-01321-7.
Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery Disease Severity and Cardiovascular Outcomes in patients with Non-ST-Segment elevation Acute Coronary Syndrome. Dis Markers. 2019. https://doi.org/10.1155/2019/6891537.
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. https://doi.org/10.1016/j.metabol.2021.154766.
Jiao Y, Su Y, Shen J, Hou X, Li Y, Wang J, et al. Evaluation of the long-term prognostic ability of triglyceride-glucose index for elderly acute coronary syndrome patients: a cohort study. Cardiovasc Diabetol. 2022;21(1):3. https://doi.org/10.1186/s12933-021-01443-y.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Triglyceride-glucose index as a surrogate marker of insulin resistance for Predicting Cardiovascular Outcomes in nondiabetic patients with Non-ST-Segment elevation Acute Coronary Syndrome undergoing percutaneous coronary intervention. J Atheroscler Thromb. 2021;28(11):1175–94. https://doi.org/10.5551/jat.59840.
Yang J, Tang YD, Zheng Y, Li C, Zhou Q, Gao J, et al. The impact of the triglyceride-glucose index on poor prognosis in NonDiabetic patients undergoing percutaneous coronary intervention. Front Endocrinol (Lausanne). 2021. https://doi.org/10.3389/fendo.2021.710240.
Drwiła D, Rostoff P, Gajos G, Nessler J, Konduracka E. Prognostic value of the triglyceride-glucose index among non-diabetic patients with acute myocardial infarction at one-year follow-up. Kardiol Pol. 2021;79(10):1116–23. https://doi.org/10.33963/KP.a2021.0104.
Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31. https://doi.org/10.1186/s12933-020-01006-7.
Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride–glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. https://doi.org/10.1186/s12933-019-0957-3.
Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr Metabolism Cardiovasc Dis. 2020;30(12):2351–62. https://doi.org/10.1016/j.numecd.2020.07.041.
Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee S-H, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):155. https://doi.org/10.1186/s12944-016-0324-2.
Gill JM. Physical activity, cardiorespiratory fitness and insulin resistance: a short update. Curr Opin Lipidol. 2007;18(1):47–52. https://doi.org/10.1097/MOL.0b013e328012b8bd.
Coats AJS, Forman DE, Haykowsky M, Kitzman DW, McNeil A, Campbell TS, et al. Physical function and exercise training in older patients with heart failure. Nat Reviews Cardiol. 2017;14(9):550–9. https://doi.org/10.1038/nrcardio.2017.70.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61 – 8. https://doi.org/10.1093/ije/dys203.
Liu LS. [2010 chinese guidelines for the management of hypertension]. Zhonghua xin xue guan bing za zhi. 2011;39(7):579–615. https://doi.org/10.3760/cma.j.issn.0253-3758.2011.07.002.
Zeng Z, Bian Y, Cui Y, Yang D, Wang Y, Yu C. Physical activity dimensions and its association with risk of diabetes in middle and older aged Chinese people. 2020;17(21):7803. https://doi.org/10.21203/rs.2.22440/v2.
Liu X, Yu S, Mao Z, Li Y, Zhang H, Yang K, et al. Dyslipidemia prevalence, awareness, treatment, control, and risk factors in chinese rural population: the Henan rural cohort study. Lipids Health Dis. 2018;17(1):119. https://doi.org/10.1186/s12944-018-0768-7.
WHO Guidelines Approved by the Guidelines Review Committee. Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. https://doi.org/10.26719/2015.21.6.379. 2010.
Chen X, Crimmins E, Hu PP, Kim JK, Meng Q, Strauss J, et al. Venous blood-based biomarkers in the China Health and Retirement Longitudinal Study: Rationale, Design, and results from the 2015 Wave. Am J Epidemiol. 2019;188(11):1871–7. https://doi.org/10.1093/aje/kwz170.
Shen Y, Zhang Y, Xiong S, Zhu X, Ke C. High-sensitivity C-reactive protein and cystatin C independently and jointly predict all-cause mortality among the middle-aged and elderly chinese population. Clin Biochem. 2019;65:7–14. https://doi.org/10.1016/j.clinbiochem.2018.12.012.
Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: insights from a general population. Nutr Metab Cardiovasc Dis. 2020;30(2):245 – 53. https://doi.org/10.1016/j.numecd.2019.09.015.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. https://doi.org/10.1002/sim.3841.
Al Jumaily T, Rose’Meyer RB, Sweeny A, Jayasinghe R. Cardiac damage associated with stress hyperglycaemia and acute coronary syndrome changes according to level of presenting blood glucose. Int J Cardiol. 2015;196:16–21. https://doi.org/10.1016/j.ijcard.2015.05.143.
Sharif S, Groenwold RHH, van der Graaf Y, Berkelmans GFN, Cramer MJ, Visseren FLJ, et al. Mediation analysis of the relationship between type 2 diabetes and cardiovascular events and all-cause mortality: Findings from the SMART cohort. Diabetes Obes Metab. 2019;21(8):1935–43. https://doi.org/10.1111/dom.13759.
Wang L, Cong H-l, Zhang J-x, Hu Y-c, Wei A, Zhang Y-y, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. https://doi.org/10.1186/s12933-020-01054-z.
Jin JL, Sun D, Cao YX, Guo YL, Wu NQ, Zhu CG, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med. 2018;50(7):576 – 86. https://doi.org/10.1080/07853890.2018.1523549.
Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–46. https://doi.org/10.21037/jtd.2018.10.79.
Gao S, Ma W, Huang S, Lin X, Yu M. Impact of triglyceride-glucose index on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metabolism Cardiovasc Dis. 2021;31(11):3184–92. https://doi.org/10.1016/j.numecd.2021.07.027.
Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62(2):345–9. https://doi.org/10.2310/jim.0000000000000044.
Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67. https://doi.org/10.1210/er.2018-00141.
Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575-85https://doi.org/10.1016/j.cmet.2011.07.015.
Reardon CA, Lingaraju A, Schoenfelt KQ, Zhou G, Cui C, Jacobs-El H, et al. Obesity and insulin resistance promote atherosclerosis through an IFNγ-Regulated macrophage protein network. Cell Rep. 2018;23(10):3021–30. https://doi.org/10.1016/j.celrep.2018.05.010.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. https://doi.org/10.1186/s12933-018-0762-4.
Levisianou D, Foussas S, Skopelitis E, Adamopoulou E, Xenopoulou T, Destounis A, et al. Arterial stiffness predicts risk for long-term recurrence in patients with type 2 diabetes admitted for acute coronary event. Diabetes Res Clin Pract. 2013;99(3):315 – 20. https://doi.org/10.1016/j.diabres.2012.11.023.
Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267–75. https://doi.org/10.1016/j.jacc.2015.03.544.
Sinnaeve PR, Steg PG, Fox KA, Van de Werf F, Montalescot G, Granger CB, et al. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute coronary events. Arch Intern Med. 2009;169(4):402–9. https://doi.org/10.1001/archinternmed.2008.572.
Adeva-Andany MM, Ameneiros-Rodríguez E, Fernández-Fernández C, Domínguez-Montero A, Funcasta-Calderón R. Insulin resistance is associated with subclinical vascular disease in humans. World J Diabetes. 2019;10(2):63–77. https://doi.org/10.4239/wjd.v10.i2.63.
Kitta Y, Nakamura T, Uematsu M, Sugamata W, Deyama J, Fujioka D, et al. Insulin resistance negatively affects long-term outcome in non-diabetic patients with coronary artery disease after therapies to reduce atherosclerotic risk factors. J Cardiol. 2013;62(6):348 – 53. https://doi.org/10.1016/j.jjcc.2013.05.006.
Dongerkery SP, Schroeder PR, Shomali ME. Insulin and its Cardiovascular Effects: what is the current evidence? Curr Diab Rep. 2017;17(12):120. https://doi.org/10.1007/s11892-017-0955-3.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Reviews Endocrinol. 2014;10(5):293–302. https://doi.org/10.1038/nrendo.2014.29.
Bastard JP, Lavoie ME, Messier V, Prud’homme D, Rabasa-Lhoret R. Evaluation of two new surrogate indices including parameters not using insulin to assess insulin sensitivity/resistance in non-diabetic postmenopausal women: a MONET group study. Diabetes Metab. 2012;38(3):258 – 63. https://doi.org/10.1016/j.diabet.2012.01.004.
Yang S-W, Park K-H, Zhou Y-J. The impact of hypoglycemia on the Cardiovascular System. :Physiology and Pathophysiology. 2016;67(9):802–9. https://doi.org/10.1177/0003319715623400.
Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res. 2016;118(7):1151–69. https://doi.org/10.1161/circresaha.116.306206.
Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12(3):144 – 53.https://doi.org/doi:https://doi.org/10.1038/nrendo.2015.216.
Angoorani P, Heshmat R, Ejtahed HS, Motlagh ME, Ziaodini H, Taheri M, et al. Validity of triglyceride-glucose index as an indicator for metabolic syndrome in children and adolescents: the CASPIAN-V study. Eat Weight Disord. 2018;23(6):877 – 83. https://doi.org/10.1007/s40519-018-0488-z.
Trifunovic D, Stankovic S, Sobic-Saranovic D, Marinkovic J, Petrovic M, Orlic D, et al. Acute insulin resistance in ST-segment elevation myocardial infarction in non-diabetic patients is associated with incomplete myocardial reperfusion and impaired coronary microcirculatory function. Cardiovasc Diabetol. 2014;13:73. https://doi.org/10.1186/1475-2840-13-73.
da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. https://doi.org/10.1186/s12933-019-0893-2.
Chiu H, Tsai H-J, Huang J-C, Wu P-Y, Hsu W-H, Lee M-Y, et al. Associations between triglyceride-glucose Index and Micro- and macro-angiopathies in type 2 diabetes Mellitus. Nutrients. 2020;12(2):328. https://doi.org/10.3390/nu12020328.
Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):155. https://doi.org/10.1186/s12944-016-0324-2.
Wen Z, Li Y, Xu L, Yue C, Wang Q, Chen R, et al. Triglyceride glucose-body Mass Index is a Reliable Indicator of Bone Mineral density and risk of osteoporotic fracture in Middle-Aged and Elderly nondiabetic chinese individuals. J Clin Med. 2022;11:19. https://doi.org/10.3390/jcm11195694.
Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7(1):e013927. https://doi.org/10.1136/bmjopen-2016-013927.
Liese AD, Roach AK, Sparks KC, Marquart L, D’Agostino RB Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the insulin resistance atherosclerosis study. Am J Clin Nutr. 2003;78(5):965 – 71. https://doi.org/10.1093/ajcn/78.5.965.
da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. https://doi.org/10.1186/s12933-019-0893-2.
Alizargar J, Hsieh NC, Wu SV. Is the use of triglyceride-glucose (TyG) index to recognize glucose disorders really practical? Eur J Pediatr. 2020;179(7):1169. https://doi.org/10.1007/s00431-020-03642-3.
Leonardi S, Gragnano F, Carrara G, Gargiulo G, Frigoli E, Vranckx P, et al. Prognostic implications of declining hemoglobin content in patients hospitalized with Acute Coronary Syndromes. J Am Coll Cardiol. 2021;77(4):375 – 88. https://doi.org/10.1016/j.jacc.2020.11.046.
Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, et al. Association of troponin levels with mortality in italian patients hospitalized with Coronavirus Disease 2019: results of a Multicenter Study. JAMA Cardiol. 2020;5(11):1274–80. https://doi.org/10.1001/jamacardio.2020.3538.
Bogle BM, Ning H, Goldberger JJ, Mehrotra S, Lloyd-Jones DM. A simple community-based risk-prediction score for Sudden Cardiac Death. Am J Med. 2018;131(5):532–9. https://doi.org/10.1016/j.amjmed.2017.12.002.
Zomer E, Owen A, Magliano DJ, Liew D, Reid C. Validation of two Framingham cardiovascular risk prediction algorithms in an australian population: the ‘old’ versus the ‘new’ Framingham equation. Eur J Cardiovasc Prev Rehabil. 2011;18(1):115 – 20. https://doi.org/10.1097/HJR.0b013e32833ace24.
Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear Associated with all-cause and Cardiovascular Mortality in the General Population. Front Cardiovasc Med. 2020;7:628109. https://doi.org/10.3389/fcvm.2020.628109.
Acknowledgements
We thank the subjects who participated in this study. We would like to thank American Journal Experts (https://www.aje.com) for English language editing.
Funding
The work was supported by the National Key R&D Program of China (Grand Nos. 2020YFC2008500 and 2020YFC2008502) and the National Natural Science Foundation of China (Grant Nos. 82202793, 81572231, 82172534, and 81501951) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC21038).
Author information
Authors and Affiliations
Contributions
LW, YW, RL and QW designed and wrote the manuscript. LX, WZ, LL, CW and CH revised the manuscript. LW and RL drew the figures. CF and QW provided critical feedback and helped to shape the manuscript. All authors listed have made a substantial contribution to the work. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Biomedical Ethics Review Committee of Peking University (protocol code IRB00001052–11015). Informed consent was obtained from all subjects involved in the study.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
The value of triglycerides, glucose, and TGI grouped by fasting state in middle-aged and old patients. Supplementary Table 2. The effect of cardiovascular-related drugs on the levels of triglycerides, glucose, and TGI. Supplementary Table 3. The difference of predictive values for triglycerides, glucose, TGI in middled-aged and old patients. Supplementary Table 4. The association between TGI and all-cause mortality grouped by different cutoff values for ages. Supplementary Table 5. Cox regression analysis for TGI levels as continuous variables predicting the risk of all-cause mortality in middle-aged and old patients. Supplementary Figure 1. The proportion of vigorous physical activity volumes in patients with different age and TGI levels. Supplementary Figure 2. The proportion of moderate physical activity volumes in patients with different age and TGI levels. Supplementary Figure 3. The proportion of mild physical activity volumes in patients with different age and TGI levels. Supplementary Figure 4. The proportion of cardiovascular mortality and non-cardiovascular mortality in middle-aged and old patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, L., Wang, Y., Liu, R. et al. Influence of age on the association between the triglyceride-glucose index and all-cause mortality in patients with cardiovascular diseases. Lipids Health Dis 21, 135 (2022). https://doi.org/10.1186/s12944-022-01738-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01738-3