Abstract
Objective
We aimed to investigate the effect of the triglyceride glucose (TyG) index on the association between diabetes and cardiovascular disease (CVD).
Methods
Data from 6,114 individuals were extracted and analyzed from the China Health and Retirement Longitudinal Survey (CHARLS) from 2011 to 2018. Logistic regression analyses were conducted to assess the relationship between diabetes and CVD across the various TyG index groups. The statistical method of subgroup analysis was used to determine the correlation between diabetes and CVD for each TyG index group by sex, history of hypertension and dyslipidemia, smoking, and drinking.
Results
Diabetes was positively associated with CVD risk after adjustment in 2011(odds ratio (OR) 1.475, 95% CI 1.243–1.752, P < 0.001). There was a gradient increase in the OR for new-onset CVD in 2018 due to diabetes at baseline across the value of the TyG index based on a fully adjusted model (P for trend < 0.05). The ORs of diabetes at baseline for CVD in 2018 were 1.657 (95% CI 0.928–2.983, P = 0.098), 1.834(95% CI 1.064–3.188, P = 0.037) and 2.234(95% CI 1.349–3.673, P = 0.006) for T1, T2 and T3 of the TyG index respectively. The gradient of increasing risk of CVD still existed among those with hypertension and nondrinkers in the subgroup analysis.
Conclusion
Elevated TyG index strengthens the correlation between diabetes mellitus and CVD in middle-aged and elderly Chinese adults.
Similar content being viewed by others
Introduction
According to the International Diabetes Federation, the prevalence of diabetes will likely increase globally from 9.3% (463 million people) in 2019 to 10.9% (700 million) in 2045 [1]. The risk of developing cardiovascular disease (CVD) has been reported to be approximately twofold higher in people with diabetes, in whom CVD is the primary cause of death [2, 3]. Therefore, better management must identify CVD development in patients with diabetes.
Insulin resistance (IR) is an abnormal metabolic state in which the target tissue has a lower response to the average insulin concentration [4, 5] and has been proposed as the critical linking factor for diabetes and cardiovascular diseases [6]. Currently, the hyperinsulinemic-euglycemic clamp is regarded as the gold standard for evaluating IR [7, 8]. However, because of the tedious and labor-intensive procedure needed, the euglycemic hyperinsulinemic clamp technique is impractical for measuring insulin sensitivity directly in large-scale epidemiological studies with limited clinical value.
The triglyceride glucose (TyG) index is a reliable alternate of IR [9]. Many studies in various countries have demonstrated that the TyG index is a crucial predictor for diabetes development [10]. Additionally, several researchers have reported a positive association between an elevated TyG index and vascular disease, including carotid atherosclerosis, arterial stiffness, and major adverse cardiovascular events (MACEs) [11,12,13,14]. The TyG index was also positively correlated with the severity of CVD and cardiovascular outcomes [15]. Unfortunately, few studies have investigated cardiovascular effects on individuals with diabetes of various TyG index levels and the interaction of the TyG index between CVD and diabetes.
In this study, a large-scale population dataset from the China Health and Retirement Longitudinal Study (CHARLS) was used to estimate whether the value of the TyG index could impact the association between diabetes and future cardiovascular disease.
Methods
Study design and population
The data for this cohort study were extracted from CHARLS, a large-scale longitudinal prospective cohort study in China enrolling adults aged 45 years or above. The objective, study design and methods of CHARLS have been described in detail elsewhere [16, 17]. In short, the CHARLS recruited 17,708 participants within 28 provinces in China, adopting a multistage probability sampling technology in the first wave (W1) between June 2011 and March 2012. All participants were Chinese, with an 81% response rate to the W1 survey. The 9,271 individuals with available baseline data from W1 and follow-up data from the fourth wave (W4, 2018) were enrolled in this study. Participants with missing data of blood tests on triglycerides (TG), fast blood glucose (FBG), and self-reported history of CVD and diabetes were excluded. Finally, after further excluding 203 participants with CVD at W1, 6,114 individuals were recruited for this study (Fig. 1). All methods were carried out in accordance with relevant guidelines and regulations. The ethical approval and experimental protocols were approved by the institutional review board of Peking University (IRB00001052–11,015). Informed consent was acquired from all participants before participation.
Data collection and definition
At baseline, trained researchers used a structured questionnaire to acquire sociodemographic status information and health-related indicators, including age, gender, educational level, smoking and drinking status. Education level was stratified as primary school or lower, junior high school and senior high school or above. Fundamental health indicators included self-reported drinking and smoking status (never, former, or current), medical condition (self-reported physician diagnosis of CVD, hypertension, diabetes, and dyslipidemia) and medication treatment for CVD, diabetes, hypertension, and dyslipidemia. Trained nurses were responsible for measuring height, weight, and diastolic and systolic blood pressure. Venous blood samples were gathered and determined for high-sensitivity C-reactive protein (hsCRP), glucose, blood urea nitrogen (BUN), cystatin C, uric acid, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), TG and creatinine by the staff of the Chinese Center for Disease Control and Prevention.
The Incidence of developing CVD events was defined as the positive answer to the question of whether the patient had doctor-diagnosed heart disease (including heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems) or stroke during the follow-up period, which was regarded as the outcome of this research. Diabetes was defined as a self-reported doctor’s diagnosis of diabetes, taking treatment of antidiabetic medications, FBG > 125 mg/dl or HbA1C > 6.5% in the blood test. The equation ln[TG(mg/dl) *FBG(mg/dl) /2] was used to calculate the value of the TyG index [18]. For covariates, estimated glomerular filtration (eGFR) was evaluated utilizing the 2009 creatinine equation according to the Chronic Kidney Disease Epidemiology Collaboration. The definition of chronic kidney disease(CKD) was eGFR < 60 mL/min/1.73 m2 [19]. Hypertension and dyslipidemia were defined as a self-reported physician diagnosis of a medical condition or being on medication [20].
Statistical analysis
Continuous variables were applied by mean (standard deviation, SD) or median (interquartile ranges, IQR) depending on normal distribution or not, while the frequency with percentage was presented for categorical variables. All participants were classified by tertiles of the TyG index (T1, T2, T3) according to the TyG index value, and the Kruskal–Wallis test, Chi-square test and ANOVA were conducted to compare differences among various TyG index tertiles as appropriate. Univariable and multivariable logistic regression analyses were conducted to estimate the relationship between DM and CVD for each TyG index group. Model 1 was a rough analysis with no adjustment to evaluate the association between diabetes and new-onset CVD in various tertiles of the TyG index. In Model 2, the analysis was adjusted for age and gender which were the most common confounding biases. In addition, complete adjusted Model 3 was calculated based on further adjustment for BMI, smoking, drinking, education, medical condition (including diabetes, hypertension, dyslipidemia, CKD), laboratory markers (including CRP, uric acid, blood urea nitrogen) and drugs against hypertension and diabetes. P for interaction was performed to estimate the tendency in the incrementing value of TyG index cohorts as the substitute of P for trend. Subgroup analyses were applied to stratify the relationship between diabetes and CVD for each TyG index group by sex, history of hypertension and dyslipidemia, smoking and drinking. In the sensitivity analysis, the above statistical analyses were performed again by substituting the TyG index, another effective indicator of insulin resistance, TG/HDL-C, to estimate the uniqueness of the trend of the TyG index.
R 4.0.3 (Vienna, Austria) was used for statistical analysis. P < 0.05 was regarded as statistically significant (two-sided).
Results
In total, 6,114 participants in CHARLS were enrolled in this cohort study. The baseline characteristics of all adults in this study based on tertiles of the TyG index value (T1-T3) and the proportion of CVD development are shown in Table 1. The mean value of the TyG index in 2011 was 8.66 (0.65). The prevalence of DM among participants in the T1, T2 and T3 groups was 3.9%, 4.2%, and 4.0%, respectively.
The baseline characteristics of participants with or without DM are shown in Table 2. During the follow-up, 753 participants (12.3%) among 6114 participants had developed new-onset CVD. Among the participants without DM, 700(11.9%) individuals developed CVD during the follow-up, while 53(21.5%) DM participants developed CVD during the follow-up.
The TyG index increased the relationship between diabetes and new-onset CVD. Overall, diabetes was positively correlated with CVD risk (OR 1.604, 95% CI 1.357–1.896, P < 0.001). After adjusting for age, sex, BUN, history of hypertension, dyslipidemia or CKD, smoking, drinking, BMI, education and medication treatments for diabetes and hypertension, diabetes was positively related to the prevalence of CVD in 2011 (OR 1.475, 95% CI 1.243–1.752, P < 0.001) and new-onset CVD risk (OR 1.437, 95% CI 1.032–1.999, P = 0.032). There was a gradient increase in the OR for new-onset CVD due to DM across the value of the TyG index based on the completely adjusted model (Table 3, Model 3, P for trend < 0.05). The ORs of diabetes for new-onset CVD were 1.657 (95% CI 0.928–2.983, P = 0.098), 1.834(95% CI 1.064–3.188, P = 0.037) and 2.234(95% CI 1.349–3.673, P = 0.006) for T1, T2 and T3, respectively. (Table 3).
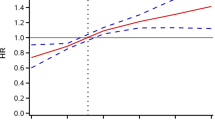
Subgroup analyses were utilized to stratify the relationship between diabetes and CVD by potential risk factors. The gradient of increasing risk of CVD still existed among those with hypertension and nondrinkers. The three-way interaction was statistically significant in hypertension (P = 0.039) and nondrinkers (P = 0.001) (Fig. 2) after complete adjustments. Stratification variables were not adjusted in the corresponding models.
The results of subgroup analyses between diabetes and CVD by TyG index cohorts after adjustment for age, sex, BMI, smoking, drinking, education, diabetes, hypertension, dyslipidemia, CKD, CRP, UA, BUN and medical treatments. Stratification variables were not adjusted in the corresponding models. CI, confidence interval; CVD, cardiovascular disease
In the sensitivity analysis, there was no apparent gradient of OR of DM in 2011 for new-onset CVD in 2018 across elevated TG/HDL-C values (T1-T3, P for trend = 0.312). The ORs of diabetes for CVD in 2018 for T1, T2, and T3 of TG/HDL-C were 1.569 (95% CI 0.876–2.810, P = 0.130), 2.195(95% CI 1.283–3.757, P = 0.004) and 1.879(95% CI 1.098–3.217, P = 0.021) respectively, after total adjustment. (Table 4).
Discussion
This study explored the effect of the TyG index in predicting future CVD risk in patients with established DM using a longitudinal cohort dataset representing the Chinese population. Even after adjusting for potential confounders, DM was associated with an elevated risk for CVD incidence, with a value of 1.475 (95% CI 1.243–1.752, P < 0.001). Most importantly, this study suggested that an increased TyG index aggravates the relationship between diabetes and future CVD.
DM is considered an established risk factor for CVD development [21]. Consistent with prior studies, we confirmed that diabetes was significantly related to increased cardiovascular risk (P < 0.001). However, the OR (1.604, 95% CI 1.357–1.896) for cardiovascular disease in adults with DM was lower than those reported in the previous cohort. This disparity is likely driven by differences in race and ethnicity [22,23,24]. For instance, Asian individuals with diabetes have lower BMI values [25]. A real-world study in China also obtained similar results (HR = 1.747, 95% CI 1.566–1.949 P < 0.0001) [26].
IR is a characteristic of diabetes [27, 28], and the TyG index is a critical marker composed of TG and FBG concentrations and a reliable and straightforward alternative to IR [29]. The TyG index was proven to be related to the risk of diabetes in many studies. A retrospective cohort study reported that a higher TyG index has a positive association with an increased incidence of diabetes in Chinese adults older than 45 years, according to the CHARLS data [30]. Another 15-years follow-up study in China showed a nonlinear relationship between the TyG index and typed two diabetes mellitus risks among the general Chinese population, and the cutoff point value was at 8.51 [10]. Compared with fast plasma glucose and triglycerides, a White European study reported that the TyG index was a better predictor of T2DM risk in normoglycemic patients [31]. However, a retrospective study from Thailand demonstrated that the TyG index appeared to be a less robust predictor of diabetes than FPG [32]. In general, the TyG index was regarded as a valuable and independent predictor for the risk of diabetes development.
Subsequently, numerous clinical studies were designed to evaluate the correlation between the TyG index and CVD in general population cohorts with and without diabetes. A Brazilian study that enrolled 2330 patients in secondary care reported that the TyG index positively correlated with high coronary artery disease (CAD) prevalence and atherosclerosis [33]. A prospective cohort study named Kailuan recruited 98,849 participants without myocardial infarction and demonstrated that an increased value of the TyG index at baseline and long-term values independently predicted the incidence of myocardial infarction [34]. Moreover, substantial changes in the TyG index were positively correlated with CVD risk observed in the 62,443 participants without CVD at baseline in China [35]. In addition, in patients who underwent percutaneous coronary intervention (PCI) after acute coronary syndrome, an elevated TyG index was independently related to in-stent restenosis after drug-eluting stent implantation in the 62,443 general population in China with some predictive value [36]. The TyG index was significantly associated with future CVD risk in the current study.
This is the first study in a prospective Chinese population evaluating the possible exacerbation of CVD by a higher TyG index level among participants with DM. The strength of this study is that we applied a sizeable national database representing middle- and older-aged Chinese individuals. Additionally, this study considered and adjusted health lifestyle habits (such as smoking and drinking status) and health status (such as hypertension and CKD) related variables and conducted subgroup analysis. Last, compared with the most recommended imaging diagnostic methods (such as myocardial perfusion scintigraphy) [37], the TyG index may be more cost-effective [38] and have broader potential application scenarios.
The results of our study need to be interpreted in consideration of its limitations. First, some unidentified medications, such as SGLT2 inhibitors, could impact the risk of CVD that could have affected the conclusions. In the current study, TG/HDL-C, another biomarker of IR, was performed in the sensitivity analyses to avoid potential bias and proved the unique trend across the tertiles to the TyG index. Second, our study sample is not ethnically representative because the CHARLS study only enrolled middle-aged to elderly adult populations in China. Future cohorts are needed to expand toward other descent.
Conclusions
Using a nationally representative sample, this study confirmed that DM is a risk factor for CVD. More importantly, the current study innovatively found that a higher TyG index aggravates the association between DM and the risk of developing CVD. These findings could provide potential clinical guidelines for DM primary prevention of future CVD due to DM. Furthermore, it is also crucial to evaluate the potential benefit of treatment targeting IR in preventing severe complications in the future.
Availability of data and materials
The data that support the findings of this study are available from the China Health and Retirement Longitudinal Study (CHARLS) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the correspondence authors Jingang Zheng upon reasonable request and with permission of CHARLS.
Abbreviations
- 95%CI:
-
95% Confidence intervals
- CVD:
-
Cardiovascular disease
- IR:
-
Insulin resistance
- TyG:
-
Triglyceride glucose
- MACE:
-
Major adverse cardiovascular events
- CHARLS:
-
China Health and Retirement Longitudinal Study
- TG:
-
Triglycerides
- FB:
-
Fast blood glucose
- TC:
-
Total cholesterol
- hsCRP:
-
High-sensitivity C-reactive protein
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- BUN:
-
Blood urea nitrogen
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration
- CKD:
-
Chronic kidney disease
- IQR:
-
Interquartile ranges
- SD:
-
Standard deviation
- CAD:
-
Coronary artery diseases
- PCI:
-
Percutaneous coronary intervention
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9<sup>th</sup> edition. Diabetes Res Clin Pract. 2019. https://doi.org/10.1016/j.diabres.2019.107843.
The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. The Lancet. 2010;375(9733):2215–22. https://doi.org/10.1016/S0140-6736(10)60484-9.
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41.
Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–48.
Krentz AJ. Insulin resistance. BMJ. 1996;313(7069):1385–9.
Pearson ER. Type 2 diabetes: a multifaceted disease. Diabetologia. 2019;62(7):1107–12.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23.
Kim JK. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol Biol. 2009;560:221–38.
Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146.
Wang Z, Zhao L, He S. Triglyceride-glucose index as predictor for future type 2 diabetes mellitus in a Chinese population in southwest China: a 15-year prospective study. Endocrine. 2021;72(1):124–31.
Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361.
Guo W, Zhu W, Wu J, Li X, Lu J, Qin P, et al. Triglyceride glucose index is associated with arterial stiffness and 10-Year cardiovascular disease risk in a chinese population. Front Cardiovasc Med. 2021;8:585776.
Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80.
Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–73.
Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-st-segment elevation acute coronary syndrome. Dis Markers. 2019;2019:6891537.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Shi Z, Nicholls SJ, Taylor AW, Magliano DJ, Appleton S, Zimmet P. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J Hypertens. 2018;36(1):54–60.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D’Agostino RB, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292(20):2495–9. https://doi.org/10.1001/jama.292.20.2495.
Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA. 2019;322(24):2389–98.
Muilwijk M, Ho F, Waddell H, Sillars A, Welsh P, Iliodromiti S, et al. Contribution of type 2 diabetes to all-cause mortality, cardiovascular disease incidence and cancer incidence in white Europeans and South Asians: findings from the UK Biobank population-based cohort study. BMJ Open Diabetes Res Care. 2019;7(1):e000765.
Bellary S, O’Hare JP, Raymond NT, Mughal S, Hanif WM, Jones A, et al. Premature cardiovascular events and mortality in south Asians with type 2 diabetes in the United Kingdom Asian Diabetes Study - effect of ethnicity on risk. Curr Med Res Opin. 2010;26(8):1873–9.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Liu Y, Li J, Dou Y, Ma H. Impacts of type 2 diabetes mellitus and hypertension on the incidence of cardiovascular diseases and stroke in China real-world setting: a retrospective cohort study. BMJ Open. 2021;11(11):e053698.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. https://doi.org/10.1038/nrendo.2014.29.
Yki-Järvinen H, Koivisto VA. natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315(4):224–30. https://doi.org/10.1056/NEJM198607243150404.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Chen CL, Liu L, Lo K, Huang JY, Yu YL, Huang YQ, et al. Association between triglyceride glucose index and risk of new-onset diabetes among Chinese adults: findings from the china health and retirement longitudinal study. Front Cardiovasc Med. 2020;7:610322.
Navarro-Gonzalez D, Sanchez-Inigo L, Pastrana-Delgado J, Fernandez-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99–105.
Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14(2):161–7.
da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira AC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89.
Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19.
Wang A, Tian X, Zuo Y, Chen S, Meng X, Wu S, et al. Change in triglyceride-glucose index predicts the risk of cardiovascular disease in the general population: a prospective cohort study. Cardiovasc Diabetol. 2021;20(1):113.
Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20(1):137.
Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguade-Bruix S, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015. https://doi.org/10.1161/CIRCIMAGING.114.002179.
Hong S, Han K, Park C-Y. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361.
Acknowledgements
We acknowledge all participants in the CHARLES research team for survey design and data collection at Peking University.
Funding
This work was supported by the National Natural Science Foundation of China (91639110), National Key Clinical Specialty Construction Project (2020-QTL-009), and Beijing Natural Science Foundation (7172195).
Author information
Authors and Affiliations
Contributions
JZ and ZY contributed to the study design. ZY, EX, SJ, YW, YT, QL and JR contributed to data collection, manuscript writing data processing and figure mapping. YG contributed to data proofread. XY and PL contributed to formal analysis; writing—original draft preparation, ZY, LY, ZG and CY contributed to review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. The ethical approvement and experimental protocols were approved by the institutional review board of Peking University (IRB00001052–11015). Informed consent has been acquired from all participants before the participation.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, Z., Xie, E., Jiao, S. et al. Triglyceride glucose index exacerbates the risk of future cardiovascular disease due to diabetes: evidence from the China Health and Retirement Longitudinal Survey (CHARLS). BMC Cardiovasc Disord 22, 236 (2022). https://doi.org/10.1186/s12872-022-02673-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02673-y