Abstract
The tripartite motif (TRIM) protein family is a highly conserved group of E3 ligases with 77 members known in the human, most of which consist of a RING-finger domain, one or two B-box domains, and a coiled-coil domain. Generally, TRIM proteins function as E3 ligases to facilitate specific proteasomal degradation of target proteins. In addition, E3 ligase independent functions of TRIM protein were also reported. In hepatocellular carcinoma, expressions of TRIM proteins are both regulated by genetic and epigenetic mechanisms. TRIM proteins regulate multiple biological activities and signaling cascades. And TRIM proteins influence hallmarks of HCC. This review systematically demonstrates the versatile roles of TRIM proteins in HCC and helps us better understand the molecular mechanism of the development and progression of HCC.
Similar content being viewed by others
Introduction
The tripartite motif (TRIM) protein family is a highly conserved group of RING-type E3 ligases with 77 members known in the human, most of which consist of a RING-finger domain, one or two B-box domains, and a coiled-coil domain [1]. Dysregulation of TRIM proteins has been found and shown crucial roles in different types of diseases including inflammation, viral infection, and cancer [2,3,4].
Liver cancer is the fourth leading cause of cancer-related death globally, and hepatocellular carcinoma (HCC) represents approximately 90% of primary liver cancer [5, 6]. In HCC, TRIM proteins have impacts on cell proliferation, apoptosis, cancer metastasis, metabolic reprogramming, stemness, carcinogenesis, immunogenicity, and resistance to cancer therapies. Furthermore, targeting TRIM proteins showed its potential effects on HCC. In this review, we summarize the roles of TRIM proteins in HCC as ubiquitin ligases or non-ubiquitination roles. We systematically demonstrate the biological functions of TRIM proteins in HCC and summarize the signaling cascades affected by TRIM proteins.
Expression pattern and biological functions of TRIM proteins in HCC
Structural classification and domain functions of TRIM proteins
The tripartite motif (TRIM) protein family is named for their highly conserved RING domain, B-box domains, and the coiled-coil (CC) region at N-terminal. Unlike N-terminal domains, C-terminal domains of TRIM proteins vary in different subtypes, and TRIM proteins can be classified into subfamily C-I to C-XI according to distinctive C-terminal domains [1]. In detail, C-terminal domains of TRIM proteins including COS domain, Fibronectin type-III domain (FN3), PRY domain, B30.2/SPRY domain (SPRY), acid-rich region (ACID), filamin-type I domain (FIL), NHL domain, PHD domain, bromodomain (BRD), Meprin and TRAF-homology domain (MATH), ADP-ribosylation factor family domain (ARF), and transmembrane region (TM). Another subfamily called UC refers to 8 TRIM proteins without RING domain (Fig. 1).
Structure of TRIM proteins. TRIM proteins are classified into subfamily C-I to C-XI according to different C-terminal domains, and a special subfamily UC without RING domain. List of abbreviation: COS domain (COS), Fibronectin type-III domain (FN3), PRY domain (PRY), B30.2/SPRY domain (SPRY), acid-rich region (ACID), filamin-type I domain (FIL), NHL domain, PHD domain, bromodomain (BRD), Meprin and TRAF-homology domain (MATH), ADP-ribosylation factor family domain (ARF), and transmembrane region (TM)
Generally, TRIM proteins are E3 ubiquitin ligases based on their RING domain [7]. B-box domains contain one or two different zinc-binding motifs and are divided into 2 types. They promote the catalytic effect of RING or mediate ubiquitination of substrates independently [8, 9]. The Coiled-coil region executes a conserved scaffolding function of forming anti-parallel homo- or hetero-dimerization [10,11,12]. In detail, members of C-IV family (TRIM7, TRIM11, TRIM22, TRIM25, TRIM26, TRIM50, TRIM65, TRIM72), C-V family (TRIM31, TRIM52), C-VI family (TRIM28, TRIM33), C-VII family (TRIM71), C-XI family (TRIM59), UC family (TRIM14) exert their roles as E3 ligases in HCC (Table 1). TRIM proteins either promote or inhibit carcinogenesis and cancer progression mainly depending on specific identifying and degrading oncogenic or tumor suppressive proteins.
N-terminal and C-terminal domains exert functions cooperatively or independently during the biological process in HCC. The SPRY helps the nuclear translocation of TRIM22 [13]. The SPRY also mediates the interaction of tyrosine-protein kinase Src (SRC) with TRIM7, the intracellular part of interferon alpha/beta receptor 1 (IFNAR1) with TRIM10, and pleckstrin homology domain leucine-rich repeats protein phosphatase 1 (PHLPP1) with TRIM11, to promote their substrate degradation [14,15,16]. Notably, TRIM14 has no RING domain, but it is still able to mediate ubiquitination degradation of NS5A through SPRY [17]. The NHL domain helps with the recognition between TRIM71 and a structural RNA stem-loop motif within the 3’-untranslated region (UTR) of CDKN1A mRNA [18]. Functions of other C-terminal domains need further research in HCC.
Expression, mutation, and regulation of TRIM proteins in HCC
Expressions of TRIM proteins are frequently altered in HCC patients (Table 2). Researches show that TRIM3, TRIM16, TRIM26, TRIM33, TRIM35, TRIM50, TRIM55, TRIM56, and TRIM58 are low-expressed in HCC samples [19,20,21,22,23,24,25,26,27]. TRIM11, TRIM14, PML, TRIM25, TRIM28, TRIM31, TRIM32, TRIM37, TRIM44, TRIM52, TRIM59, TRIM66, and TRIM71 are high-expressed in HCC samples [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Interestingly, TRIM7 and TRIM29 exhibit opposite tendencies according to different studies. The difference may be the results of HBV infection status, tumor stages, or different detecting technology [14, 43,44,45].
Genetic alterations for TRIM proteins are common. We explore the genetic alteration events for TRIM proteins in 353 HCC patients through TCGA in the cbioportal [46, 47]. TRIMs are altered in 56% (196/353) of patients. TRIM11(7%), TRIM17(6%), TRIM35(6%), TRIM46(12%), TRIM55(7%), TRIM58(8%), TRIM67(7%), and TRIML1(5%) exhibit significantly higher mutation rate (Additional file 1: Fig. S1). TRIM proteins are also associated with common genetic mutations in HCC. We select top5 mutated genes according to TCGA-LIHC (TP53, TTN, CTNNB1, MUC16, and ALB) together with 10 genes impacting common pathways in HCC (AXIN1, APC, IRF2, CDKN2A, ARID1A, ARID2, KRAS, PIK3CA, RPS6KA3, NFE2L2) [48]. We explore the differential gene expression under different mutation statuses in HCC through TIMER2.0 [49]. The most linked genes are TP53(35/76), CTNNB1(25/76), and AXIN1(24/76) (Additional file 2: Table S1).
Apart from genetic variations, expressions of TRIM proteins are also modulated via epigenetic mechanisms, including DNA methylation, mi-RNA, circRNA, and long non-coding RNAs (lncRNA). TRIM21 is down-regulated by methylation in its 5′-UTR [50]. TRIM33 is reduced through aberrant CpG methylation at its promoter [22].
Mi-RNAs regulate gene expression by inhibiting transcription or inducing decay of mRNAs. Transcript of TRIM23 is targeted by miR-194, whose inhibition is a key process in NF-κB activation [51]. High-expressed miR-837 inhibits TRIM25 expression in HCC [52]. MiR-424-5p acts as a tumor suppressor by targeting TRIM29 [45]. MiR-29c-3p down-regulates TRIM31 expression [53]. MiR-4417 down-regulates TRIM35 [54]. And miR-4698 down-regulates TRIM59 [55]. Furthermore, circRNAs can act as sponges of miRNAs to repress miRNA function. Circ_0091579 acts as a sponge of miR-136-5p to up-regulate TRIM27 [56]. Circular RNA PVT1 (CircPVT1) acts as a sponge of miR-377 to up-regulate TRIM23 and promote HCC [57]. Hsa-circ-0026134 acts as a sponge of miR-127-5p to down-regulate TRIM25 [58]. Besides, tripartite motifs containing 33-derived circRNA (circTRIM33-12) acts as a sponge of miR-191 to up-regulate Methylcytosine dioxygenase TET1 (TET1) and prevents HCC progression [59].
LncRNA XIST directly targets miR-192 to up-regulate TRIM25 [60]. LncRNA rhophilin Rho GTPase binding protein 1 antisense RNA 1 (RHPN1-AS1) promotes TRIM16 expression [61]. LncRNA RP11-286H15.1 binds to poly(A) binding protein 4 (PABPC4) and promotes its ubiquitin degradation. while PABPC4 could enhance TRIM37 mRNA stabilization [62].
Prognostic roles of TRIM proteins in HCC
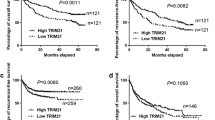
Dysregulation of TRIM proteins significantly influences the prognosis of HCC. The hazard ratios (HR), case numbers, p-values, type of survival (overall survival (OS), recurrence-free survival (RFS)), and associated clinical characteristics are recruited in Table 2. We have also made a cox regression analysis for all TRIM proteins based on TCGA-LIHC patients with OS for more than a month (Additional file 2: Table S2) (Additional file 3: Fig. S2).
Besides, bioinformatic mining revealed that TRIM28, TRIM37, TRIM45, and TRIM59 could serve as efficient biomarkers in predicting OS, PFS, DSS, and RFS based on TCGA [63, 64]. Another study built a TRIM gene-based signature (including TRIM5, MID1(TRIM18), TRIM21, TRIM32, TRIM44, and TRIM47), which shows decent efficiency in predicting OS [HR: 6.630 (3.030–14.504), p < 0.001] in TCGA and GSE76427. A nomogram based on risk score, age, and TNM stage also showed better discrimination in predicting OS [65].
Furthermore, combinations of TRIM proteins with other proteins show higher efficiency in clinical assessments. Our study found that the combination of TRIM33 and phosphorylated SMAD2 is more efficient in predicting recurrence and OS in HCC [22]. TRIM28/minichromosomal maintenance complex component 6 (MCM6) is a novel marker for diagnosing HCC [66]. Zinc finger protein 354C (ZNF354C)/TRIM28/HDAC6 and TRIM35/pyruvate kinase isoform M2 (PKM2) are more effective prognostic factors for HCC [23, 67].
TRIM proteins exert epigenetic regulations in HCC
Roles in chromatin remodeling
Members of the C-VI subfamily (TRIM24, TRIM28, and TRIM33) showed regulator effects on chromatin remodeling. TRIM28 is the scaffold of chromatin-remodeling complexes, consisting of the histone methyltransferase SET domain bifurcated 1 (SETDB1), histone deacetylases (HDACs), nuclear remodeling factors, and heterochromatin protein 1 (HP1) [68]. TRIM28 is recruited to the DNA via its interaction with Krüppel-associated box zinc finger proteins (KRAB) and synergistically inhibits KRAB-suppressed genes like endogenous retro-viruses (ERV) family [69, 70]. HP1, especially HP1β, mediates specific retrotransposons silent partly through TRIM28, whose dysregulation in KRAB/TRIM28/SETDB1 complex is determinant in HP1-dependent hepatic tumorigenesis (Fig. 2) [71]. LINC00624 promotes ubiquitin degradation of HDAC6 and interacts with TRIM28 to inhibit the conjugation between ZNF354C and TRIM28, therefore disrupting the HDAC6-TRIM28-ZNF354C corepressor complex formation and eliminating transcription suppression of Chromodomain-helicase-DNA-binding protein 1-like (CHD1L) and lymphoma 9-like protein (BCL9) [67]. Aging and obesity strengthen TRIM28-dependent epigenetic regulation, such as activating tumor-associated molecular patterns (TAMPs), dampening the farnesoid x receptor (FXR) pathway, over-activating β-catenin, and altering the androgen pathway [72]. Besides, SETDB1 and its cofactor TRIM28 physically interact with METTL3 (methyltransferase-like 3) in mouse embryonic stem cells, which shows TRIM proteins may participate in N6-methyladenosine (m6A) methylation of mRNA [73].
TRIM proteins in chromatin remodeling. TRIM28 is the scaffold for chromatin-remodeling complex, which comprises the SETDB1, HDACs, nuclear remodeling factors, and HP1. LINC00624 decrease the trimerization of TRIM28, HDAC6, and ZNF354C through hindering the interaction between TRIM28 and ZNF354C, and promoting TRIM28-mediated ubiquitin degradation of HDAC6. The dimers formed of TRIM24 and TRIM33 are recruited to the RARE at the LTR, which leads to the silencing of RA signaling or VL30 and prevents HCC. SETDB1 SET domain bifurcated 1; HDAC histone deacetylases; HP1 heterochromatin protein 1; ZNF354C Zinc finger protein 354C; RARE retinoic acid–responsive elements; LTR long terminal repeats; RA retinoic acid; VL30 virus-like 30S-class ERVs
Similarly, TRIM24 and TRIM33 form chromatin remodeling complexes with HP1 and HDAC (Fig. 2) [74, 75]. TRIM24 and TRIM33 are recruited to the retinoic acid-responsive elements (RARE) at the long terminal repeats (LTRs) to inhibit the expression of RA receptor α (RARα)-mediated transcription, signal transducer, activator of transcription 1 (STAT1), or virus-like 30S-class ERVs (VL30) [76, 77]. Activation of the VL30 elicits chronic inflammation and generates enhancer RNAs to promote transcription of TRIM24 and neighboring genes [77]. Interestingly, the KRAB-zinc finger proteins (ZFPs)-TRIM28 system could also affect VL30 in mouse embryonic fibroblasts [78]. It is worth further investigating whether TRIM28 regulates VL30 synergistically with TRIM24 and TRIM33 in HCC.
Roles in mRNA instability
TRIM71, a member of the C-VII subfamily, disrupts miRNA-mediated gene silencing through mediating ubiquitin degradation of proteins argonaute (Ago) 1 and Ago2 to decrease RNA-induced silencing complex (RISC). Thus, TRIM71 inhibits functions of tumor-suppressive miR-let-7 and oncogenic miR-21 in HCC [42, 79]. However, a recent study contradicted that TRIM71 proceeds degradations of mRNA through non-canonical nonsense-mediated decay (NMD) rather than interfering RISC. TRIM71 destabilizes p21 mRNA through cooperating with NMD factors serine/threonine-protein kinase SMG1 (SMG1), SMG7, and regulator of nonsense transcripts 1 (UPF1) [18].
Effects of TRIM proteins on hallmarks of carcinogenesis
Sustaining proliferation
Sustaining proliferation is a hallmark of cancer. Aberrant expression of TRIM proteins leads to abnormal cell cycle progression and sustaining proliferation.
Our previous study revealed that TRIM33 suppresses the TGF-β pathway by mediating ubiquitin degradation of mothers against decapentaplegic homolog 4 (Smad4) and inhibiting the Smad2/3/4 complex formation. TRIM33 suppressed TGF-β down-stream p21, p15, and restored c-myc. Therefore, TRIM33 promotes proliferation in early-phase HCC [22]. TRIM52 mediates ubiquitin degradation of protein phosphatase, Mg2+/Mn2+ dependent 1A (PPM1A), which dephosphorylates Smad2 and Smad3 to inhibit the TGF-β pathway and results in increased expression of TP53 and p21 [39].
Cell cycle defect is a common feature of human cancers, whose central part is the harmonious control of cyclin-dependent kinases (CDKs), cyclin-dependent kinase inhibitors (CKIs), and cyclins [80, 81]. TRIM proteins regulate certain CDKs and CKIs to induce G1/S cell cycle arrest in HCC. TRIM28 interacts with Ubiquitin-conjugating enzyme E2 S (UBE2S) in the nucleus. They enhance p27 ubiquitination to mediate G1/S arrest, which can be blocked by cephalomannine [82]. TRIM65 interacts with AXIN1 and promotes ubiquitin degradation of AXIN1 to activate the β-catenin pathway and promote expressions of cyclin D1 and c-myc. TRIM65 is up-regulated by HMGA1 [83]. And TRIM71 suppresses functions of p21 mRNA through NMD [18]. P53 is mainly activated by the PML-IV isoform in PML-nuclear bodies (PML-NBs) [84]. TRIM11 is associated with decreased TP53, p21, p27 expression and increased cyclin D1 expression [85]. TRIM59 promotes ubiquitin degradation of protein phosphatase 1B (PPM1B), which dephosphorylates and inactivates CDK2. TRIM59 also decreases cyclin E1, CDK6, and CDK1, and degrades TP53 [40, 86]. TRIM7 facilitates ubiquitin degradation of Dual specificity protein phosphatase 6 (DUSP6) to inhibit downstream inactivation of p38 and MAPK, and TRIM7 is associated with high TP53 and p21 expressions [43]. In addition, TRIM3 induces G0/G1 cycle arrest [87]. Associations between TRIM and other phases of cell cycle in HCC need further research.
Mutation of myc is significantly deleterious to HCC development, abnormal activation of myc-related signaling is crucial for the proliferation of HCC. Mitogen-activated protein kinase kinase kinase 13 (MAP3K13) promotes phosphorylation and suppresses proteasomal degradation of TRIM25. TRIM25 mediates the ubiquitin degradation of F-box/WD repeat-containing protein 7α (FBXW7α), which is the main E3 ligase that down-regulates c-myc [88]. TRIM56 is associated with up-regulated c-Myc and activated β-catenin [26]. TRIM71 inhibits functions of miR-let-7 and up-regulated down-stream c-Myc, Lin-28B, HMGA2 and type 1 insulin-like growth factor receptor (IGF1R) [42].
Resistant to apoptosis
Apoptosis is a process of programmed cell death that can be triggered by either the extrinsic or the intrinsic pathways [89]. PML induces apoptosis in parvovirus H-1 or HCV-infected HCC cells [90, 91]. PML induces apoptosis through P53, Fas, TNF, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and caspase pathways [91,92,93,94]. Knockdown of TRIM44 decreases the expression of cellular inhibitors of apoptosis 1 (c-IAP1), c-IAP2, and XIAP, which are anti-apoptosis targets in the NF-κB signaling pathway [38, 95].
Abnormal antioxidative response via aberrant Kelch-like ECH-associated protein 1 (Keap1)- nuclear factor erythroid 2-related factor 2 (NRF2) signaling is a common event in the progression of HCC [96]. TRIM25 promotes ubiquitin degradation of Keap1, therefore over-activating NRF2 to alleviate oxidative stress and reduce apoptosis in HCC [97].
Anoikis is defined as the detached from the extracellular matrix (ECM) -induced apoptosis, and resistance to anoikis is a hallmark of cancer [98]. TRIM31 targets TP53 proteasomal degradation to over-activates the AMP-activated protein kinase (AMPK) pathway to promote anoikis [99]. Oppositely, TRIM50 down-regulates SNAIL through ubiquitin degradation, therefore reverses EMT and inhibits anoikis resistance [24].
Metastasis of HCC
The dysregulated Wnt/β-catenin pathway is a common event in HCC tumorigenesis and is related to stemness and aggressive phenotype of HCC [100]. TRIM66 facilitates glycogen synthase kinase-3 beta (GSK-3β) phosphorylation and thereby inhibits the β-catenin pathway [101]. Inversely, TRIM37 strengthens pS9GSK-3β expression, which is the inactive type of GSK3β [36]. TRIM29 inhibits the expression and phosphorylation of β-catenin to prevent metastasis [44, 45]. TRIM28 inhibits the activation of β-catenin triggered by aging and obesity [72]. TRIM65 and TRIM37 suppress metastasis through activating the β-catenin pathway [36, 62, 83].
Epithelial–mesenchymal transition (EMT) refers to the process that epithelial cells acquire mesenchymal features, which promotes invasion and metastasis in cancer [102]. TRIM11 interacts with PHLPP1 by SPRY domain and mediates proteasomal degradation of PHLPP1. Thereby it promotes the phosphorylation and activation of AKT. Thus, TRIM11 significantly promotes migration, invasion, and EMT [16, 103]. TRIM33 inhibits down-stream targets of TGF-β pathway, including MMP1, CXCR4, Snail, and Slug, to promote invasion and metastasis in end-phase HCC [22]. TRIM50 mediates ubiquitin degradation of SNAIL to reverse EMT [24]. TRIM16 suppresses zinc finger E-box-binding homeobox 2 (ZEB2) expression to down-regulate E-cadherin [20].
MMP proteins facilitate the degradation of ECM proteins. MMP2 and MMP9 are major MMPs in the pathogenesis of EMT in HCC [104]. TRIM55 is associated decreases expression of MMP2 and vimentin [25]. TRIM66 decreases MMP9 expression [41]. Spermatogenesis associated serine rich 2 (SPATS2) promotes HCC progression via the TRIM44-p-STAT3 axis. TRIM44 is associated with expressions of hypoxia-inducible factor 1α (HIF-1α), MMP9, serine/threonine-protein kinase pim-1 (PIM-1), and BCL-2 [105].
TRIM25 mediates ubiquitination of metastasis associated 1 protein (MTA1) at K98 to suppress metastasis in HCC [106, 107].
TRIM7 interacts with SRC and affects the SRC-mTORC1-ribosomal protein S6 kinase beta-1 (S6K1) axis [14]. TRIM31 mediates ubiquitin degradation of the TSC1-TSC2 complex to over-activate mTORC1 [34]. Therefore, TRIM7 and TRIM31 result in unresolved ER stress, autophagy suppression, and invasion.
Metabolic reprogramming of HCC cells
The Warburg effect refers to the metabolic reprogramming in cancer that energy is generated through aerobic glycolysis instead of mitochondrial oxidative phosphorylation [108]. PKM2 is a rate-limiting enzyme in glycolysis, whose phosphorylation provides extra metabolic advantages for HCC cells. TRIM35 competes with fibroblast growth factor receptor 1 (FGFR1) to interact with PKM2 and consequently inhibits the phosphorylation of PKM2 [23, 109]. TRIM28/melanoma-associated antigen (MAGE)-A3/MAGE-C2 complex promotes the Warburg effect through ubiquitin degradation of fructose-1,6-bisphosphatase 1 (FBP1), which is the rate-limiting enzyme in gluconeogenesis [110]. TRIM11 is significantly induced upon glucose deprivation. TRIM11 down-regulates AMPKβ2 through ubiquitin degradation to suppress AMPK pathway and leads to starvation-induced autophagy [111].
Furthermore, TRIM proteins are involved in lipid, hormone, and biliary acid metabolism. Liver-specific TRIM28-knockout mice exhibit aberrant androgen receptor stimulation, biliary acid disturbances, and significantly altered gut microbiota such as Prevotella, Akkermansia muciniphila, and Bacteroides uniformis, which are species predominantly associated with metabolic dysfunction and inflammation. Notably, this abnormality can be completely abolished under axenic conditions [72]. Liver-specific TRIM28-knockout results in sexual dimorphic metabolic syndrome through activating the ERK1/2-MAPK pathway. Loss of TRIM28-dependent epigenetic silencing results in activation of fat-specific protein 27 (FSP27), glutathione S-transferase, Cyp2d9, Cyp2a, Cyp2b, and Cyp3a gene clusters, and thereby leads to male-predominant liver steatosis and adenoma [112].
PML-knockout mice show increased white fat initially, but exhibit weight loss and white fat browning in end-stage HCC with the metabolic reprogramming from glycogen storage to lipolysis [113]. PML-deficient HBsAg-transgenic mice showed obvious oxidative phosphorylation and fatty acid metabolism impairments and encountered early steatosis-specific liver tumorigenesis [114].
Besides, TRIM8 promotes phosphorylation of TGF-β–activated kinase 1 (TAK1) [115]. TRIM16 down-regulates phosphorylated TAK1 [116]. They regulate downstream c-Jun N-terminal kinase/p38 in hepatocytes and influence steatohepatitis progression, which may point out future studies in HCC.
Initiation of HCC
Somatic hepatocyte-specific inactivation of TRIM24, TRIM28, or TRIM33 all promotes spontaneous HCC [74, 117]. TRIM24 forms quantities of dimers with TRIM33, and a few trimers with TRIM28 and TRIM33. Liver TRIM24-knockout induced HCC is significantly promoted by further loss of TRIM33, and is slightly hindered by further loss of TRIM28 [74]. Mechanistically, TRIM24 attenuates RARα-mediated transcription through chromatin remodeling as mentioned above. Thus, TRIM24 deficiency activates downstream targets of the RA pathway such as Cyp26a1, protein-glutamine gamma-glutamyl transferase 2 (TGM2), RBP1, and receptors for retinol uptake STRA6 (STRA6). Notably, deletion of a single allele of RARα is sufficient to restore the phenotype of TRIM24-knockout mice [117]. TRIM24 also binds to the RARE of STAT1 promoter to inhibit STAT1 expression and promotes expressions of tumor-suppressive factors such as p21, Bmyc, and hepatocyte nuclear factor 6 (HNF6) [76].
Tumor initiation cells (TICs) in HCC are a subset of HCC cells with stem cell features and influence the initiation, cell growth, drug resistance, and recurrence of tumors [118]. Arsenite treatment represses PML expression, which down-regulates Oct4, Sox2, and Klf4 expressions. As a result, it reduces viability and stemness of CD133+ CD13+ TICs and enhances sensitivity to pirarubicin in HCC [119].
Immune responses
Anti-HBV effect
HBV- and HCV infection are leading risk factors for HCC worldwide, and dysregulated responses to the infection of HBV or HCV fuel the progression of HCC [120,121,122].
Multiple TRIM proteins are identified inhibiting HBV replication in HCC, including TRIM5, TRIM6, TRIM11, TRIM14, TRIM25, TRIM26, TRIM31, and TRIM41 [123]. TRIM25 is an interferon-stimulated gene (ISG) augmented by IFN and IL-27, which mediate lysine 63-ubiquitination of RIG-I to suppress HBV replication [124]. PML is significantly associated with genomic instability and DNA repair in HBV-related HCC [125, 126]. PML is negatively correlated with HBsAg because of proteasomal degradation or translocation of HBsAg to the nucleus [113, 114, 125, 126]. PML suppresses the early phase of HCC since it enhances DNA repair and induces resistance to IFN-α or DNA damage-induced apoptosis (Fig. 3A), but turns out to be oncogenic in the end stage. It enhances a metabolic shift from glycogen storage to lipolysis, which implicates more energy available for driving HCC progression (Fig. 3B) [113, 125].
TRIM proteins in anti-HBV response of HCC. A PML is negatively correlated with HBsAg at the early-phase, because of proteasomal degradation and translocation to the nuclear. Down-regulated PML promotes apoptosis resistance and impairs DNA repair in HCC. B Long-term suppressed PML results in genome instability, which may confront the loss of HBV genes and HBsAg expression. However, PML turns out to be oncogenic since it enhances metabolic shift from glycogen storage to lipolysis. C TRIM22 is up-regulated under IFN stimulations. IFN activate IRF1, which is transferred to the nucleus and promote TRIM22 transcription by conjugating to its CpG island. D HBx suppresses IFN-induced transcription of TRIM22 gene through a single CpG methylation in its 5′-UTR, which reduces the IRF1 binding, thereby suppressing the IFN-stimulated induction of TRIM22 and exhibiting HBV immune escape. IRF1 IFN regulatory factor-1; HBx HBV regulatory protein X; UTR untranslated region
HBV regulatory protein X (HBx) stimulates HBV gene expression from the covalently closed circular (cccDNA) and is involved in HCC development [127]. TRIM14 is a STAT1-dependent type-I ISG. The TRIM14 SPRY domain interacts with the C-terminal of HBx to inhibit the formation of Smc-HBx-damage-specific DNA-binding protein 1 (DDB1) complex [128]. TRIM5γ is another type-I ISG which mediates HBx ubiquitin degradation through the B-box domain. TRIM31 is recruited by TRIM5γ and can also mediate HBx ubiquitin degradation [129]. Another study supplemented that TRIM31 is a type-III ISG and can be induced upon HBV replication [130].
HBx also regulates TRIM expressions. TRIM22 can be strongly stimulated by IFN-α and IFN-γ through IFN regulatory factor-1 (IRF1) in HCC. TRIM22 suppresses HBV core promoter by its nuclear-located RING domain, whose translocation is mediated by the SPRY domain (Fig. 3C) [13]. However, HBx protein down-regulates the transcription of TRIM22 through a single CpG methylation in its 5’-UTR to inhibit the binding between the promoter and IRF1, thereby inhibiting the IFN-stimulated induction of TRIM22 and resulting in HCC (Fig. 3D) [50]. And HBx protein promotes expressions of TRIM7 or TRIM52 [43, 131].
Besides, TRIM proteins may regulate STAT3 to regulate IFN responses. PML suppresses interleukin (IL)-6-induced Tyr705 and Ser727 phosphorylation of STAT3 and interferes with the interaction between STAT3 and HDAC3 to suppress the IL-6/STAT [132]. TRIM10 inhibits the association between non-receptor tyrosine-protein kinase TYK2 (TYK2) and IFNAR1 to inhibit the IFN/JAK/STAT pathway [15].
Anti-HCV effect
PML declines in HCV transgenic mice and develops more spontaneous or phenobarbital/diethylnitrosamine (DEN) induced HCC via down-regulating RNA NLRP12, Ras association domain-containing protein 6 (RASSF6), and TRAIL expressions [133,134,135]. HCV core protein interacts and inactivates PML-IV in PML-NBs to inhibit phosphorylation and acetylation of P53, which leads to dysregulation of Fas in HCC [91, 136]. IFN-α can compensate for the expression of PML suppressed by HCV core protein [94]. TRIM14 is an ISG and inhibits HCV infection by SPRY domain-dependent targeted degradation of NS5A protein [17, 137]. Similarly, TRIM22 mediates ubiquitin degradation of NS5A under IFN-α treatment to inhibit HCV replication [138]. TRIM26 expedites HCV replication in HCC through its interaction with HCV-encoded NS5B and mediating K27-linked ubiquitination of NS5B to promote NS5B-NS5A interaction [139].
Tumor microenvironment (TME)
The TME exists abundant of tumor cells as well as innate and adaptive immune cells, stromal cells, endothelial cells, and cancer-associated fibroblasts. Exploring of TME may help develop the treatment strategies in HCC [140]. We collected the associations between TRIM family and Immune Infiltrates in TIMER database (Fig. 4) [141, 142]. TRIM59, PML(TRIM19), TRIM46, and MEFV (TRIM20) significantly affect these immune infiltrations.
Correlation between TRIM and immune infiltrates in LIHC. Correlation between TRIM and abundance of 6 immune infiltrates in LIHC using TIMER database. The purity-corrected partial Spearman’s rho values are displayed with statistical significance (p < 0.05) marked with *. TRIM59, PML(TRIM19), TRIM46, and MEFV(TRIM20) significantly affect these immune infiltrations
TRIM28 activated by Receptor-interacting serine/threonine-protein kinase 3 (RIPK3) loses its chromatin binding ability. Thus, RIPK3 transactivates NF-κB and SOX9, strengthens CD8+ T cell and DC maturation [143]. TRIM28 and SETDB1 form regulatory complex, whose loss could activate cGAS–STING innate immunity to strengthen antitumor effects of anti–PD-L1 [144]. Verteporfin inhibits PD-L1 through the STAT1-IRF1-TRIM28 signaling axis [145]. However, none of these results focus on HCC.
Resistance to cancer therapies
Sorafenib is recommended as first/second line systematic therapy for BCLC B or C stage HCC patients [6]. TRIM72 interacts with Ras-related C3 botulinum toxin substrate 1 (RAC1) with its Coiled-coil domain. It down-regulates RAC1 through ubiquitin degradation and inhibits the RAC1-MAPK pathway to enhance sensitivity to sorafenib [146]. TRIM62 promotes phosphorylation of IKKβ and NF-κB p65 nuclear translocation. Therefore, TRIM62 activates the NF-κB pathway and induces sorafenib resistance [147]. TRIM37 activates the AKT pathway to induce sorafenib resistance [37].
Besides, TRIM25 strengthens sensitivity to epirubicin through promoting ubiquitin degradation of PTEN [32]. TRIM32 induces oxaliplatin resistance [35]. High-expressed TRIM44 accelerates doxorubicin resistance by activating the NF-κB pathway [38]. Arsenic trioxide (ATO) is a traditional chemotherapeutic drug for HCC patients [148]. ATO suppresses HCC formation synergistically with PML through promoting TP53, Bcl-2, and strengthening PML-NBs expression and functions [149]. However, PML also down-regulates the aldehyde dehydrogenase family 3 member A1 (ALDH3A1) by physically combining with the promoter to induce ATO resistance [30].
Prospect
Currently, the poor prognosis and low percentage of patients responding to systemic therapies are characteristics of HCC, and new therapeutic methods for targeting HCC are urgently needed. As TRIM proteins exert functions mainly through the ubiquitin system (UPS), it seems feasible that use proteasomal inhibitors to block TRIM proteins to ameliorate HCC. Proteasome inhibitors like bortezomib, ixazomib, and carfilzomib have shown effectiveness in some cancer, but their applications are unsatisfying in HCC, as bortezomib in HCC in phase II trial lacked activity [150,151,152]. Carfilzomib and gankyrin inhibitors are far from clinical applications [153]. Several factors account for the ineffectiveness in common. Bortezomib may not inhibit the UPS in the liver as expected, or the dose and schedule need further modulations. Alternatively, the crosstalk of intertwined signaling pathways may counteract each other. Side effects of proteasome inhibitors like neuropathy also restrain their application [154].
Recently, proteolysis-targeting chimeras (PROTACs) give novel insight into applications of TRIM proteins. PROTACs technology employs E3 ligase ligands and fuses target protein with E3 ligase by a flexible chemical bond, to elicit ectopic ubiquitination and degrade specific target proteins [155]. PROTACs entered clinical research for cancer therapies in 2019 [156]. And the first oral PROTAC ARV-110 has shown effectiveness in prostate cancer [157]. TRIM proteins have promising applications in HCC through two aspects of PROTACs. TRIM proteins can be direct targets of PROTACs. For instance, dTRIM24 can recruit VHL E3-ligase to elicit potent and selective degradation of TRIM24 [158]. And dTRIM24 has successfully degraded TRIM24 in human metaplastic breast cancer patient-derived xenografts to decrease tumor cell viability [159]. TRIM proteins may also become mediums in PROTACs, which means recruiting TRIM proteins to specifically down-regulate some oncoproteins to alleviate HCC. But the design of new PROTACs ligand compounds is challenging since they need to conjugate the “right binding site” for limited ubiquitin sites as well as for reserving enough space to elongate the ubiquitin chain.
Notably, many virus proteins enable to hijack host E3 ligases to antagonize anti-viral factors, which may enlighten the development of PROTACs of TRIM proteins. These proteins seem natural and prototypical PROTACs [156, 160]. The HPV E6 oncoprotein employs ubiquitin-specific protease 15 (USP15) to degrade TRIM25 [161]. Murine gamma herpesvirus 68 induces proteasomal-dependent degradation of PML by the virion tegument protein ORF75c [162].
In addition, the C-VI subfamily (TRIM24, TRIM28, and TRIM33) might be the best transitional therapeutic targets in the future. This subfamily has powerful influences on the progression of HCC. Homozygous deletion of any of them leads to spontaneous HCC, and they regulate epigenetic silencing through chromatin remodeling. They affect diverse signaling pathways including the RA pathway, β-catenin pathway, TGF-β pathway, et.al. On the other hand, the PROTAC dTRIM24 has been invented. RA may also restrain the function of TRIM24. This may also enlighten our next-step transitional research.
Besides, there are still some unsolved problems in the TRIM family. The distribution is tightly linked to protein functions. We collect the intracellular location of TRIM proteins from UniProt (Additional file 2: Table S3) [163]. But few researchers concerning about the sublocation of TRIM in HCC. Another shortness is the relationship between TRIM and first- or second-line therapy drugs for HCC. It is worth further investigating whether TRIM may benefit our current therapies in HCC. As lncRNAs are essential in regulating expressions and functions of TRIM proteins, it seems better to investigate relationships between lncRNA and TRIM on m6A regulations or chemotherapy resistance in HCC.
Conclusion
Growing clinical research has revealed that expressions of TRIM proteins are frequently altered and significantly associated with clinical indexes and prognosis in HCC. Some TRIM proteins are novel tumor markers and independent prognostic factors for HCC, indicating their potential in early diagnoses, prognosis assessments, and clinical therapies. In HCC, TRIM proteins regulate their proliferation, apoptosis, metastasis, metabolic reprogramming, immune responses, and resistance to cancer therapies. Mechanistically, TRIM proteins regulate levels and functions of downstream proteins through ubiquitination-dependent and independent mechanisms, and specific members of TRIM proteins regulate the activity of TGF-β/Smad, MAPK, PI3K-AKT, Wnt/β-catenin, cell cycle, STATs, and RA signaling cascades in HCC (Fig. 5). Targeting TRIM proteins showed therapeutic potential in HCC.
Signaling pathways that regulated by TRIM proteins. TRIM proteins affect multiple signaling cascades, including TGF-β pathway, cell cycle pathway, AKT/mTOR pathway, Wnt/β-catenin pathway, MAPK pathway, IFN/STAT pathway, and RA pathway. They mediate ubiquitin degradation of key proteins in pathways to activate or inactivate these signaling pathways
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- TRIM:
-
Tripartite motif
- AKT:
-
Protein kinase B
- mTOR:
-
Mammalian target of rapamycin
- MAPK:
-
MAP kinase
- RING:
-
RING-finger domain
- B1 or B2:
-
B-box domain
- CC:
-
Coiled-coil region
- COS:
-
COS domain
- FN3:
-
Fibronectin type-III domain
- PRY:
-
PRY domain
- SPRY:
-
B30.2/SPRY domain
- ACID:
-
Acid-rich region
- FIL:
-
Filamin-type I G domain
- NHL:
-
NHL domain
- PHD:
-
PHD domain
- BRD:
-
Bromodomain
- MATH:
-
Meprin and TRAF-homology domain
- ARF:
-
ADP-ribosylation factor family domain
- TM:
-
Transmembrane region
- SRC:
-
Proto-oncogene tyrosine-protein kinase Src
- IFNAR1:
-
Interferon alpha/beta receptor 1
- IFN:
-
Interferon
- CDKN1A:
-
P21
- SETDB1:
-
Histone methyltransferase SET domain bifurcated 1
- HDAC:
-
Histone deacetylase
- HP1:
-
Heterochromatin protein 1
- KRAB:
-
Krüppel-associated box zinc finger proteins
- ERV:
-
Endogenous retro-viruses
- ZNF354C:
-
Zinc finger protein 354C
- BCL9:
-
B-cell CLL/lymphoma 9 protein
- CHD1L:
-
Chromo-domain-helicase-DNA-binding protein 1-like
- FSP27:
-
Fat-specific protein 27
- Cyp:
-
Cytochrome p450
- TAMPs:
-
Tumor-associated molecular patterns
- FXR:
-
Dampening the farnesoid x receptor
- LTRs:
-
Long terminal repeats
- RARE:
-
Retinoic acid–responsive elements
- RA:
-
Retinoic acid
- STAT:
-
Signal transducer and activator of transcription
- VL30:
-
Virus-like 30S ERV
- ZFP:
-
Zinc finger proteins
- miRNA:
-
MicroRNA
- AGO1:
-
Protein argonaute
- RISC:
-
RNA-induced silencing complex
- IGF1R:
-
Type 1 insulin-like growth factor receptor
- NMD:
-
Nonsense-mediated decay
- PTC:
-
Premature termination codon
- UPF1:
-
Nonsense transcripts 1
- UTR:
-
Untranslated region
- SMAD:
-
Mothers against decapentaplegic homolog
- PPM1A:
-
Protein phosphatase, Mg2+/Mn2+ dependent 1A
- PI3K:
-
Phosphoinositide 3-kinase
- PTEN:
-
Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN
- PHLPP1:
-
Pleckstrin homology domain leucine-rich repeats protein phosphatase 1
- TSC1:
-
Hamartin
- TSC2:
-
Tuberin
- S6K1:
-
Ribosomal protein S6 kinase beta-1
- GSK-3β:
-
Glycogen synthase kinase-3 beta
- MMP:
-
Matrix metalloproteinase
- RBM24:
-
RNA-binding protein 24
- HMGA1:
-
High mobility group A1
- CDKs:
-
Cyclin-dependent kinases
- CKI:
-
Cyclin-dependent kinase inhibitors
- UBE2S:
-
Ubiquitin-conjugating enzyme E2 S
- PML:
-
TRIM19, promyelocytic leukemia
- PML-NBs:
-
PML-nuclear bodies
- PPM1B:
-
Protein phosphatase 1B
- ERK:
-
Extracellular signal-related kinases
- MAP3K13:
-
Mitogen-activated protein kinase kinase kinase 13
- FBXW7α:
-
F-box/WD repeat-containing protein 7α
- DUSPs:
-
Dual specificity phosphatases
- ROS:
-
Reactive oxygen species
- Keap1:
-
Kelch-like ECH-associated protein 1
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- RARα:
-
Retinoic acid receptor α
- HNF6:
-
Hepatocyte nuclear factor 6
- IL:
-
Interleukin
- SPATS2:
-
Spermatogenesis associated serine rich 2
- HIF-1α:
-
Hypoxia-inducible factor 1α
- PIM-1:
-
Serine/threonine-protein kinase pim-1
- TYK2:
-
Non-receptor tyrosine-protein kinase TYK2
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- TTR:
-
The median time to recurrence
- AFP:
-
α-Fetoprotein
- MCM6:
-
Minichromosomal maintenance complex component 6
- PKM2:
-
Pyruvate kinase isoform M2
- p27:
-
Cyclin-dependent kinase inhibitor 1B
- p15/ink4b:
-
Cyclin-dependent kinase 4 inhibitor B
- LncRNA:
-
Long non-coding RNA
- PABPC4:
-
Poly(A) binding protein 4
- Lin-28B:
-
Protein lin-28 homolog B
- IGF1R:
-
Insulin-like growth factor 1 receptor
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- AMPK:
-
Monophosphate-activated protein kinase
- c-IAP1:
-
Cellular inhibitor of apoptosis 1
- EMT:
-
Epithelial–mesenchymal transition
- ZEB2:
-
Zinc finger E-box-binding homeobox 2
- MTA1:
-
Metastasis associated 1 protein
- circTRIM33-12:
-
Tripartite motifs containing 33-derived circRNA
- FGFR1:
-
Fibroblast growth factor receptor
- MAGE:
-
Melanoma-associated antigen
- FBP1:
-
Fructose-1,6-bisphosphatase 1
- FSP27:
-
Fat-specific protein 27
- TAK1:
-
TGF-β-activated kinase 1
- CSCs:
-
Liver cancer stem cells
- IRF1:
-
IFN regulatory factor-1
- ISG:
-
Interferon-stimulated gene
- DEN:
-
Diethylnitrosamine
- RASSF6:
-
Ras association domain-containing protein 6
- ATO:
-
Arsenic trioxide
- ALDH3A1:
-
Aldehyde dehydrogenase 3 family member A1
- UPS:
-
Ubiquitin system
- PROTACs:
-
Proteolysis-targeting chimeras
- USP15:
-
Ubiquitin-specific protease 15
References
Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42(4):297–311.
Yang L, Xia H. TRIM proteins in inflammation: from expression to emerging regulatory mechanisms. Inflammation. 2021;44(3):811–20.
Shen Z, Wei L, Yu ZB, Yao ZY, Cheng J, Wang YT, Song XT, Li M. The roles of TRIMs in antiviral innate immune signaling. Front Cell Infect Microbiol. 2021;11: 628275.
Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11(11):792–804.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. The tripartite motif family identifies cell compartments. EMBO J. 2001;20(9):2140–51.
Massiah MA, Simmons BN, Short KM, Cox TC. Solution structure of the RBCC/TRIM B-box1 domain of human MID1: B-box with a RING. J Mol Biol. 2006;358(2):532–45.
Tao H, Simmons BN, Singireddy S, Jakkidi M, Short KM, Cox TC, Massiah MA. Structure of the MID1 tandem B-boxes reveals an interaction reminiscent of intermolecular ring heterodimers. Biochemistry. 2008;47(8):2450–7.
Li Y, Wu H, Wu W, Zhuo W, Liu W, Zhang Y, Cheng M, Chen YG, Gao N, Yu H, Wang L, Li W, Yang M. Structural insights into the TRIM family of ubiquitin E3 ligases. Cell Res. 2014;24(6):762–5.
Napolitano LM, Meroni G. TRIM family: pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life. 2012;64(1):64–71.
Sanchez JG, Okreglicka K, Chandrasekaran V, Welker JM, Sundquist WI, Pornillos O. The tripartite motif coiled-coil is an elongated antiparallel hairpin dimer. Proc Natl Acad Sci USA. 2014;111(7):2494–9.
Gao B, Duan Z, Xu W, Xiong S. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology. 2009;50(2):424–33.
Zhu L, Qin C, Li T, Ma X, Qiu Y, Lin Y, Ma D, Qin Z, Sun C, Shen X, Zhao Y, Han L. The E3 ubiquitin ligase TRIM7 suppressed hepatocellular carcinoma progression by directly targeting Src protein. Cell Death Differ. 2020;27(6):1819–31.
Guo M, Cao W, Chen S, Tian R, Wang L, Liu Q, Zhang L, Wang Z, Zhao M, Lu Q, Zhu H. TRIM10 binds to IFN-alpha/beta receptor 1 to negatively regulate type I IFN signal transduction. Eur J Immunol. 2021;51(7):1762–73.
Yang J, Ye J, Ma T, Tang F, Huang L, Liu Z, Tian S, Cheng X, Zhang L, Guo Z, Tu F, He M, Xu X, Lu X, Wu Y, Zeng X, Zou J, Wang X, Peng W, Zhang P. Tripartite motif-containing protein 11 promotes hepatocellular carcinogenesis through ubiquitin-proteasome-mediated degradation of pleckstrin homology domain leucine-rich repeats protein phosphatase 1. Hepatology. 2021;76(3):612–29.
Wang S, Chen Y, Li C, Wu Y, Guo L, Peng C, Huang Y, Cheng G, Qin FX. TRIM14 inhibits hepatitis C virus infection by SPRY domain-dependent targeted degradation of the viral NS5A protein. Sci Rep. 2016;6:32336.
Torres-Fernandez LA, Jux B, Bille M, Port Y, Schneider K, Geyer M, Mayer G, Kolanus W. The mRNA repressor TRIM71 cooperates with nonsense-mediated decay factors to destabilize the mRNA of CDKN1A/p21. Nucleic Acids Res. 2019;47(22):11861–79.
Chao J, Zhang XF, Pan QZ, Zhao JJ, Jiang SS, Wang Y, Zhang JH, Xia JC. Decreased expression of TRIM3 is associated with poor prognosis in patients with primary hepatocellular carcinoma. Med Oncol (Northwood, London, England). 2014;31(8):102.
Li L, Dong L, Qu X, Jin S, Lv X, Tan G. Tripartite motif 16 inhibits hepatocellular carcinoma cell migration and invasion. Int J Oncol. 2016;48(4):1639–49.
Wang Y, He D, Yang L, Wen B, Dai J, Zhang Q, Kang J, He W, Ding Q, He D. TRIM26 functions as a novel tumor suppressor of hepatocellular carcinoma and its downregulation contributes to worse prognosis. Biochem Biophys Res Commun. 2015;463(3):458–65.
Ding ZY, Jin GN, Wang W, Chen WX, Wu YH, Ai X, Chen L, Zhang WG, Liang HF, Laurence A, Zhang MZ, Datta PK, Zhang B, Chen XP. Reduced expression of transcriptional intermediary factor 1 gamma promotes metastasis and indicates poor prognosis of hepatocellular carcinoma. Hepatology. 2014;60(5):1620–36.
Chen Z, Lu X, Wang Z, Jin G, Wang Q, Chen D, Chen T, Li J, Fan J, Cong W, Gao Q, He X. Co-expression of PKM2 and TRIM35 predicts survival and recurrence in hepatocellular carcinoma. Oncotarget. 2015;6(4):2538–48.
Ma X, Ma X, Qiu Y, Zhu L, Lin Y, You Y, Ma D, Qin Z, Sun C, Zhao Y, Sun Y, Han L. TRIM50 suppressed hepatocarcinoma progression through directly targeting SNAIL for ubiquitous degradation. Cell Death Dis. 2018;9(6):608.
Li X, Huang L, Gao W. Overexpression of tripartite motif conaining 55 (TRIM55) inhibits migration and invasion of hepatocellular carcinoma (HCC) cells via epithelial-mesenchymal transition and matrix metalloproteinase-2 (MMP2). Med Sci Monit. 2019;25:771–7.
Yang Y, Mao FF, Guo L, Guo WX. TRIM56 suppresses the malignant development of hepatocellular carcinoma via targeting RBM24 and inactivating the Wnt signaling. Eur Rev Med Pharmacol Sci. 2021;25(2):722–30.
Qiu X, Huang Y, Zhou Y, Zheng F. Aberrant methylation of TRIM58 in hepatocellular carcinoma and its potential clinical implication. Oncol Rep. 2016;36(2):811–8.
Chen Y, Li L, Qian X, Ge Y, Xu G. High expression of TRIM11 correlates with poor prognosis in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2017;41(2):190–6.
Dong B, Zhang W. High levels of TRIM14 are associated with poor prognosis in hepatocellular carcinoma. Oncol Res Treat. 2018;41(3):129–34.
Zhang X, Yang XR, Sun C, Hu B, Sun YF, Huang XW, Wang Z, He YF, Zeng HY, Qiu SJ, Cao Y, Fan J, Zhou J. Promyelocytic leukemia protein induces arsenic trioxide resistance through regulation of aldehyde dehydrogenase 3 family member A1 in hepatocellular carcinoma. Cancer Lett. 2015;366(1):112–22.
Chan JY, Chin W, Liew CT, Chang KS, Johnson PJ. Altered expression of the growth and transformation suppressor PML gene in human hepatocellular carcinomas and in hepatitis tissues. Eur J Cancer (Oxford, England 1990). 1998;34(7):1015–22.
Yuan P, Zheng A, Tang Q. Tripartite motif protein 25 is associated with epirubicin resistance in hepatocellular carcinoma cells via regulating PTEN/AKT pathway. Cell Biol Int. 2020;44(7):1503–13.
Wang Y, Jiang J, Li Q, Ma H, Xu Z, Gao Y. KAP1 is overexpressed in hepatocellular carcinoma and its clinical significance. Int J Clin Oncol. 2016;21(5):927–33.
Guo P, Ma X, Zhao W, Huai W, Li T, Qiu Y, Zhang Y, Han L. TRIM31 is upregulated in hepatocellular carcinoma and promotes disease progression by inducing ubiquitination of TSC1-TSC2 complex. Oncogene. 2018;37(4):478–88.
Cui X, Lin Z, Chen Y, Mao X, Ni W, Liu J, Zhou H, Shan X, Chen L, Lv J, Shen Z, Duan C, Hu B, Ni R. Upregulated TRIM32 correlates with enhanced cell proliferation and poor prognosis in hepatocellular carcinoma. Mol Cell Biochem. 2016;421(1–2):127–37.
Jiang J, Yu C, Chen M, Tian S, Sun C. Over-expression of TRIM37 promotes cell migration and metastasis in hepatocellular carcinoma by activating Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2015;464(4):1120–7.
Tan G, Xie B, Yu N, Huang J, Zhang B, Lin F, Li H. TRIM37 overexpression is associated with chemoresistance in hepatocellular carcinoma via activating the AKT signaling pathway. Int J Clin Oncol. 2021;26(3):532–42.
Zhu X, Wu Y, Miao X, Li C, Yin H, Yang S, Lu X, Liu Y, Chen Y, Shen R, Chen X, He S. High expression of TRIM44 is associated with enhanced cell proliferation, migration, invasion, and resistance to doxorubicin in hepatocellular carcinoma. Tumour Biol. 2016;37(11):14615–28.
Zhang Y, Tao R, Wu SS, Xu CC, Wang JL, Chen J, Yu YS, Tang ZH, Chen XH, Zang GQ. TRIM52 up-regulation in hepatocellular carcinoma cells promotes proliferation, migration and invasion through the ubiquitination of PPM1A. J Exp Clin Cancer Res CR. 2018;37(1):116.
Ying H, Ji L, Xu Z, Fan X, Tong Y, Liu H, Zhao J, Cai X. TRIM59 promotes tumor growth in hepatocellular carcinoma and regulates the cell cycle by degradation of protein phosphatase 1B. Cancer Lett. 2020;473:13–24.
Zhang HG, Pan YW, Feng J, Zeng CT, Zhao XQ, Liang B, Zhang WW. TRIM66 promotes malignant progression of hepatocellular carcinoma by inhibiting E-cadherin expression through the EMT pathway. Eur Rev Med Pharmacol Sci. 2019;23(5):2003–12.
Chen YL, Yuan RH, Yang WC, Hsu HC, Jeng YM. The stem cell E3-ligase Lin-41 promotes liver cancer progression through inhibition of microRNA-mediated gene silencing. J Pathol. 2013;229(3):486–96.
Hu X, Tang Z, Ma S, Yu Y, Chen X, Zang G. Tripartite motif-containing protein 7 regulates hepatocellular carcinoma cell proliferation via the DUSP6/p38 pathway. Biochem Biophys Res Commun. 2019;511(4):889–95.
Xu M, Hu J, Zhou B, Zhong Y, Lin N, Xu R. TRIM29 prevents hepatocellular carcinoma progression by inhibiting Wnt/beta-catenin signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2019;51(1):68–77.
Du H, Xu Q, Xiao S, Wu Z, Gong J, Liu C, Ren G, Wu H. MicroRNA-424-5p acts as a potential biomarker and inhibits proliferation and invasion in hepatocellular carcinoma by targeting TRIM29. Life Sci. 2019;224:1–11.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4.
Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, Clement B, Balabaud C, Chevet E, Laurent A, Couchy G, Letouze E, Calvo F, Zucman-Rossi J. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44(6):694–8.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER20 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–14.
Lim KH, Park ES, Kim DH, Cho KC, Kim KP, Park YK, Ahn SH, Park SH, Kim KH, Kim CW, Kang HS, Lee AR, Park S, Sim H, Won J, Seok K, You JS, Lee JH, Yi NJ, Lee KW, Suh KS, Seong BL, Kim KH. Suppression of interferon-mediated anti-HBV response by single CpG methylation in the 5′-UTR of TRIM22. Gut. 2018;67(1):166–78.
Bao C, Li Y, Huan L, Zhang Y, Zhao F, Wang Q, Liang L, Ding J, Liu L, Chen T, Li J, Yao M, Huang S, He X. NF-κB signaling relieves negative regulation by miR-194 in hepatocellular carcinoma by suppressing the transcription factor HNF-1α. Sci Signal. 2015;8(387):ra75.
Li YH, Zhong M, Zang HL, Tian XF. The E3 ligase for metastasis associated 1 protein, TRIM25, is targeted by microRNA-873 in hepatocellular carcinoma. Exp Cell Res. 2018;368(1):37–41.
Lv T, Jiang L, Kong L, Yang J. MicroRNA-29c-3p acts as a tumor suppressor gene and inhibits tumor progression in hepatocellular carcinoma by targeting TRIM31. Oncol Rep. 2020;43(3):953–64.
Song L, Zhang W, Chang Z, Pan Y, Zong H, Fan Q, Wang L. miR-4417 targets tripartite motif-containing 35 (TRIM35) and regulates pyruvate kinase muscle 2 (PKM2) phosphorylation to promote proliferation and suppress apoptosis in hepatocellular carcinoma cells. Med Sci Monit. 2017;23:1741–50.
Yu D, Zhu L, Tu H, Wu L, Yang H, Xu C. miR-4698-Trim59 axis plays a suppressive role in hepatocellular carcinoma. Front Biosci (Landmark edition). 2020;25:1120–31.
Mao Y, Ding Z, Jiang M, Yuan B, Zhang Y, Zhang X. Circ_0091579 exerts an oncogenic role in hepatocellular carcinoma via mediating miR-136–5p/TRIM27. Biomed J. 2021. https://doi.org/10.1016/j.bj.2021.12.009.
Bu N, Dong Z, Zhang L, Zhu W, Wei F, Zheng S. CircPVT1 regulates cell proliferation, apoptosis and glycolysis in hepatocellular carcinoma via miR-377/TRIM23 Axis. Cancer Manag Res. 2020;12:12945–56.
Zhang W, Zhu L, Yang G, Zhou B, Wang J, Qu X, Yan Z, Qian S, Liu R. Hsa_circ_0026134 expression promoted TRIM25- and IGF2BP3-mediated hepatocellular carcinoma cell proliferation and invasion via sponging miR-127–5p. 2020. Biosci Rep. https://doi.org/10.1042/BSR20191418.
Zhang PF, Wei CY, Huang XY, Peng R, Yang X, Lu JC, Zhang C, Gao C, Cai JB, Gao PT, Gao DM, Shi GM, Ke AW, Fan J. Circular RNA circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol Cancer. 2019;18(1):105.
Wang J, Yin G, Bian H, Yang J, Zhou P, Yan K, Liu C, Chen P, Zhu J, Li Z, Xue T. LncRNA XIST upregulates TRIM25 via negatively regulating miR-192 in hepatitis B virus-related hepatocellular carcinoma. Mol Med. 2021;27(1):41.
Bai Z, Li H, Li C, Sheng C, Zhao X. Integrated analysis identifies a long non-coding RNAs-messenger RNAs signature for prediction of prognosis in hepatitis B virus-hepatocellular carcinoma patients. Medicine. 2020;99(40): e21503.
Jiang X, Wang G, Liu Y, Mei C, Yao Y, Wu X, Chen X, Ma W, Li K, Zhang Z, Yuan Y. A novel long non-coding RNA RP11–286H15.1 represses hepatocellular carcinoma progression by promoting ubiquitination of PABPC4. Cancer Lett. 2021;499:109–21.
Wu L, Yin X, Jiang K, Yin J, Yu H, Yang L, Ma C, Yan S. Comprehensive profiling of the TRIpartite motif family to identify pivot genes in hepatocellular carcinoma. Cancer Med. 2022;11(7):1712–31.
Su H, Tang Y, Nie K, Wang Z, Wang H, Dong H, Chen G. Identification prognostic value and correlation with tumor-infiltrating immune cells of tripartite-motif family genes in hepatocellular carcinoma. Int J Gen Med. 2022;15:1349–63.
Dai W, Wang J, Wang Z, Xiao Y, Li J, Hong L, Pei M, Zhang J, Yang P, Wu X, Tang W, Jiang X, Jiang P, Xiang L, Li A, Lin J, Liu S, Wang J. Comprehensive analysis of the prognostic values of the TRIM family in hepatocellular carcinoma. Front Oncol. 2021;11: 767644.
Jia W, Xie L, Wang X, Zhang Q, Wei B, Li H, Qin S, Chen S, Liu J, Tan Y, Zheng S, Liang X, Yang X. The impact of MCM6 on hepatocellular carcinoma in a Southern Chinese Zhuang population. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2020;127: 110171.
Li Z, Lu X, Liu Y, Zhao J, Ma S, Yin H, Huang S, Zhao Y, He X. Gain of LINC00624 enhances liver cancer progression by disrupting the histone deacetylase 6/tripartite motif containing 28/zinc finger protein 354C corepressor complex. Hepatology. 2020;73(5):1764–82.
Iyengar S, Farnham PJ. KAP1 protein: an enigmatic master regulator of the genome. J Biol Chem. 2011;286(30):26267–76.
O’Geen H, Squazzo SL, Iyengar S, Blahnik K, Rinn JL, Chang HY, Green R, Farnham PJ. Genome-wide analysis of KAP1 binding suggests autoregulation of KRAB-ZNFs. PLoS Genet. 2007;3(6): e89.
Yang P, Wang Y, Macfarlan TS. The role of KRAB-ZFPs in transposable element repression and mammalian evolution. Trends Genet. 2017;33(11):871–81.
Saksouk N, Hajdari S, Perez Y, Pratlong M, Barrachina C, Graber C, Grégoire D, Zavoriti A, Sarrazin A, Pirot N, Noël JY, Khellaf L, Fabbrizio E, Julien E, Cammas FM. The mouse HP1 proteins are essential for preventing liver tumorigenesis. Oncogene. 2020;39(13):2676–91.
Cassano M, Offner S, Planet E, Piersigilli A, Jang SM, Henry H, Geuking MB, Mooser C, McCoy KD, Macpherson AJ, Trono D. Polyphenic trait promotes liver cancer in a model of epigenetic instability in mice. Hepatology. 2017;66(1):235–51.
Xu W, Li J, He C, Wen J, Ma H, Rong B, Diao J, Wang L, Wang J, Wu F, Tan L, Shi YG, Shi Y, Shen H. METTL3 regulates heterochromatin in mouse embryonic stem cells. Nature. 2021;591(7849):317–21.
Herquel B, Ouararhni K, Khetchoumian K, Ignat M, Teletin M, Mark M, Béchade G, Van Dorsselaer A, Sanglier-Cianférani S, Hamiche A, Cammas F, Davidson I, Losson R. Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc Natl Acad Sci USA. 2011;108(20):8212–7.
Nielsen AL, Ortiz JA, You J, Oulad-Abdelghani M, Khechumian R, Gansmuller A, Chambon P, Losson R. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 1999;18(22):6385–95.
Tisserand J, Khetchoumian K, Thibault C, Dembele D, Chambon P, Losson R. Tripartite motif 24 (Trim24/Tif1alpha) tumor suppressor protein is a novel negative regulator of interferon (IFN)/signal transducers and activators of transcription (STAT) signaling pathway acting through retinoic acid receptor alpha (Raralpha) inhibition. J Biol Chem. 2011;286(38):33369–79.
Herquel B, Ouararhni K, Martianov I, Le Gras S, Ye T, Keime C, Lerouge T, Jost B, Cammas F, Losson R, Davidson I. Trim24-repressed VL30 retrotransposons regulate gene expression by producing noncoding RNA. Nat Struct Mol Biol. 2013;20(3):339–46.
Kato M, Takemoto K, Shinkai Y. A somatic role for the histone methyltransferase Setdb1 in endogenous retrovirus silencing. Nat Commun. 2018;9(1):1683.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–66.
Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14(8):518–28.
Zhang RY, Liu ZK, Wei D, Yong YL, Lin P, Li H, Liu M, Zheng NS, Liu K, Hu CX, Yang XZ, Chen ZN, Bian H. UBE2S interacting with TRIM28 in the nucleus accelerates cell cycle by ubiquitination of p27 to promote hepatocellular carcinoma development. Signal Transduct Target Ther. 2021;6(1):64.
Yang YF, Zhang MF, Tian QH, Zhang CZ. TRIM65 triggers β-catenin signaling via ubiquitylation of Axin1 to promote hepatocellular carcinoma. J Cell Sci. 2017;130(18):3108–15.
Guo A, Salomoni P, Luo J, Shih A, Zhong S, Gu W, Pandolfi PP. The function of PML in p53-dependent apoptosis. Nat Cell Biol. 2000;2(10):730–6.
Liu J, Rao J, Lou X, Zhai J, Ni Z, Wang X. Upregulated trim11 exerts its oncogenic effects in hepatocellular carcinoma through inhibition of P53. Cell Physiol Biochem. 2017;44(1):255–66.
Sun G, Sui X, Han D, Gao J, Liu Y, Zhou L. TRIM59 promotes cell proliferation, migration and invasion in human hepatocellular carcinoma cells. Pharmazie. 2017;72(11):674–9.
Huang XQ, Zhang XF, Xia JH, Chao J, Pan QZ, Zhao JJ, Zhou ZQ, Chen CL, Tang Y, Weng DS, Zhang JH, Xia JC. Tripartite motif-containing 3 (TRIM3) inhibits tumor growth and metastasis of liver cancer. Chin J Cancer. 2017;36(1):77.
Zhang Q, Li X, Cui K, Liu C, Wu M, Prochownik EV, Li Y. The MAP3K13-TRIM25-FBXW7alpha axis affects c-Myc protein stability and tumor development. Cell Death Differ. 2020;27(2):420–33.
Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21(11):678–95.
Sieben M, Herzer K, Zeidler M, Heinrichs V, Leuchs B, Schuler M, Cornelis JJ, Galle PR, Rommelaere J, Moehler M. Killing of p53-deficient hepatoma cells by parvovirus H-1 and chemotherapeutics requires promyelocytic leukemia protein. World J Gastroenterol. 2008;14(24):3819–28.
Herzer K, Weyer S, Krammer PH, Galle PR, Hofmann TG. Hepatitis C virus core protein inhibits tumor suppressor protein promyelocytic leukemia function in human hepatoma cells. Can Res. 2005;65(23):10830–7.
Cambiaghi V, Giuliani V, Lombardi S, Marinelli C, Toffalorio F, Pelicci PG. TRIM proteins in cancer. Adv Exp Med Biol. 2012;770:77–91.
Son SH, Yu E, Choi EK, Lee H, Choi J. Promyelocytic leukemia protein-induced growth suppression and cell death in liver cancer cells. Cancer Gene Ther. 2005;12(1):1–11.
Herzer K, Hofmann TG, Teufel A, Schimanski CC, Moehler M, Kanzler S, Schulze-Bergkamen H, Galle PR. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocytic leukemia protein and TRAIL independently of p53. Can Res. 2009;69(3):855–62.
Lin MT, Chang CC, Chen ST, Chang HL, Su JL, Chau YP, Kuo ML. Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J Biol Chem. 2004;279(23):24015–23.
Brahma MK, Gilglioni EH, Zhou L, Trepo E, Chen P, Gurzov EN. Oxidative stress in obesity-associated hepatocellular carcinoma: sources, signaling and therapeutic challenges. Oncogene. 2021;40(33):5155–67.
Liu Y, Tao S, Liao L, Li Y, Li H, Li Z, Lin L, Wan X, Yang X, Chen L. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat Commun. 2020;11(1):348.
Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer. 2014;14(9):632–41.
Guo P, Qiu Y, Ma X, Li T, Ma X, Zhu L, Lin Y, Han L. Tripartite motif 31 promotes resistance to anoikis of hepatocarcinoma cells through regulation of p53-AMPK axis. Exp Cell Res. 2018;368(1):59–66.
Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59(5):1107–17.
Fan W, Du F, Liu X. TRIM66 confers tumorigenicity of hepatocellular carcinoma cells by regulating GSK-3β-dependent Wnt/β-catenin signaling. Eur J Pharmacol. 2019;850:109–17.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–26.
Zhang Z, Xu C, Zhang X, Huang L, Zheng C, Chen H, Wang Y, Ju H, Yao Q. TRIM11 upregulation contributes to proliferation, invasion, and EMT of hepatocellular carcinoma cells. Oncol Res. 2017;25(5):691–9.
Scheau C, Badarau IA, Costache R, Caruntu C, Mihai GL, Didilescu AC, Constantin C, Neagu M. The role of matrix metalloproteinases in the epithelial–mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol (Amst). 2019;9423907:2019.
Chen L, Yi C, Li W, Tseng Y, Zhang J, Liu J. Inhibition of SPATS2 suppresses proliferation and invasion of hepatocellular carcinoma cells through TRIM44-STAT3 signaling pathway. J Cancer. 2021;12(1):89–98.
Li YH, Zhong M, Zang HL, Tian XF. Mechanism of TRIM25 mediated ubiquitination of metastasis associated protein (MTA) 1 in normal liver cells. Exp Cell Res. 2018;371(1):250–4.
Zang HL, Ren SN, Cao H, Tian XF. The ubiquitin ligase TRIM25 inhibits hepatocellular carcinoma progression by targeting metastasis associated 1 protein. IUBMB Life. 2017;69(10):795–801.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Chen Z, Wang Z, Guo W, Zhang Z, Zhao F, Zhao Y, Jia D, Ding J, Wang H, Yao M, He X. TRIM35 Interacts with pyruvate kinase isoform M2 to suppress the Warburg effect and tumorigenicity in hepatocellular carcinoma. Oncogene. 2015;34(30):3946–56.
Jin X, Pan Y, Wang L, Zhang L, Ravichandran R, Potts PR, Jiang J, Wu H, Huang H. MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis. 2017;6(4): e312.
Liu Y, Xu Y, Wang F, Tong Y, Li H, Wan X, Yang X, Chen L. Inhibition of AMPK activity by TRIM11 facilitates cell survival of hepatocellular carcinoma under metabolic stress. Clin Transl Med. 2021;11(12): e617.
Bojkowska K, Aloisio F, Cassano M, Kapopoulou A, Santoni de Sio F, Zangger N, Offner S, Cartoni C, Thomas C, Quenneville S, Johnsson K, Trono D. Liver-specific ablation of Krüppel-associated box-associated protein 1 in mice leads to male-predominant hepatosteatosis and development of liver adenoma. Hepatology. 2012;56(4):1279–90.
Chung YL, Wu ML. Dual oncogenic and tumor suppressor roles of the promyelocytic leukemia gene in hepatocarcinogenesis associated with hepatitis B virus surface antigen. Oncotarget. 2016;7(19):28393–407.
Chung YL, Wu ML. The role of promyelocytic leukemia protein in steatosis-associated hepatic tumors related to chronic hepatitis B virus infection. Transl Oncol. 2018;11(3):743–54.
Yan FJ, Zhang XJ, Wang WX, Ji YX, Wang PX, Yang Y, Gong J, Shen LJ, Zhu XY, Huang Z, Li H. The E3 ligase tripartite motif 8 targets TAK1 to promote insulin resistance and steatohepatitis. Hepatology. 2017;65(5):1492–511.
Wang L, Zhang X, Lin ZB, Yang PJ, Xu H, Duan JL, Ruan B, Song P, Liu JJ, Yue ZS, Fang ZQ, Hu H, Liu Z, Huang XL, Yang L, Tian S, Tao KS, Han H, Dou KF. Tripartite motif 16 ameliorates nonalcoholic steatohepatitis by promoting the degradation of phospho-TAK1. Cell Metab. 2021;33(7):1372–88.
Khetchoumian K, Teletin M, Tisserand J, Mark M, Herquel B, Ignat M, Zucman-Rossi J, Cammas F, Lerouge T, Thibault C, Metzger D, Chambon P, Losson R. Loss of Trim24 (Tif1alpha) gene function confers oncogenic activity to retinoic acid receptor alpha. Nat Genet. 2007;39(12):1500–6.
Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma-from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;19(1):26–44.
Tang H, Jin Y, Jin S, Tan Z, Peng Z, Kuang Y. Arsenite inhibits the function of CD133(+) CD13(+) liver cancer stem cells by reducing PML and Oct4 protein expression. Tumour Biol. 2016;37(10):14103–15.
Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 Suppl):S84–101.
Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol. 2009;51(4):810–20.
Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, Wang MC, Zhou WP. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: a randomized controlled trial. Ann Surg. 2018;268(6):943–54.
Zhang S, Guo JT, Wu JZ, Yang G. Identification and characterization of multiple TRIM proteins that inhibit hepatitis B virus transcription. PLoS ONE. 2013;8(8): e70001.
Tan G, Xiao Q, Song H, Ma F, Xu F, Peng D, Li N, Wang X, Niu J, Gao P, Qin FX, Cheng G. Type I IFN augments IL-27-dependent TRIM25 expression to inhibit HBV replication. Cell Mol Immunol. 2018;15(3):272–81.
Chung YL. Defective DNA damage response and repair in liver cells expressing hepatitis B virus surface antigen. FASEB J. 2013;27(6):2316–27.
Chung YL, Wu ML. Promyelocytic leukaemia protein links DNA damage response and repair to hepatitis B virus-related hepatocarcinogenesis. J Pathol. 2013;230(4):377–87.
Riviere L, Quioc-Salomon B, Fallot G, Halgand B, Feray C, Buendia MA, Neuveut C. Hepatitis B virus replicating in hepatocellular carcinoma encodes HBx variants with preserved ability to antagonize restriction by Smc5/6. Antiviral Res. 2019;172: 104618.
Tan G, Xu F, Song H, Yuan Y, Xiao Q, Ma F, Qin FX, Cheng G. Identification of TRIM14 as a type I IFN-stimulated gene controlling hepatitis B virus replication by targeting HBx. Front Immunol. 2018;9:1872.
Tan G, Yi Z, Song H, Xu F, Li F, Aliyari R, Zhang H, Du P, Ding Y, Niu J, Wang X, Su L, Qin FX, Cheng G. Type-I-IFN-stimulated gene TRIM5gamma inhibits HBV replication by promoting HBx degradation. Cell Rep. 2019;29(11):3551–63.
Xu F, Song H, Xiao Q, Wei Q, Pang X, Gao Y, Tan G. Type-III interferon stimulated gene TRIM31 mutation in an HBV patient blocks its ability in promoting HBx degradation. Virus Res. 2022;308: 198650.
Zhang Y, Wu SS, Chen XH, Tang ZH, Yu YS, Zang GQ. Tripartite motif containing 52 (TRIM52) promotes cell proliferation in hepatitis B virus-associated hepatocellular carcinoma. Med Sci Monit. 2017;23:5202–10.
Kato M, Muromoto R, Togi S, Iwakami M, Kitai Y, Kon S, Oritani K, Matsuda T. PML suppresses IL-6-induced STAT3 activation by interfering with STAT3 and HDAC3 interaction. Biochem Biophys Res Commun. 2015;461(2):366–71.
Straub K, Husen P, Baba HA, Trippler M, Wedemeyer H, Herzer K. Promyelocytic leukemia protein deficiency leads to spontaneous formation of liver tumors in hepatitis C virus transgenic mice. Cancer Med. 2019;8(8):3793–802.
Herzer K, Carbow A, Sydor S, Sowa JP, Biesterfeld S, Hofmann TG, Galle PR, Gerken G, Canbay A. Deficiency of the promyelocytic leukemia protein fosters hepatitis C-associated hepatocarcinogenesis in mice. PLoS ONE. 2012;7(9): e44474.
Yoon GS, Yu E. Overexpression of promyelocytic leukemia protein and alteration of PML nuclear bodies in early stage of hepatocarcinogenesis. J Korean Med Sci. 2001;16(4):433–8.
Fogal V, Gostissa M, Sandy P, Zacchi P, Sternsdorf T, Jensen K, Pandolfi PP, Will H, Schneider C, Del Sal G. Regulation of p53 activity in nuclear bodies by a specific PML isoform. EMBO J. 2000;19(22):6185–95.
Metz P, Dazert E, Ruggieri A, Mazur J, Kaderali L, Kaul A, Zeuge U, Windisch MP, Trippler M, Lohmann V, Binder M, Frese M, Bartenschlager R. Identification of type I and type II interferon-induced effectors controlling hepatitis C virus replication. Hepatology. 2012;56(6):2082–93.
Yang C, Zhao X, Sun D, Yang L, Chong C, Pan Y, Chi X, Gao Y, Wang M, Shi X, Sun H, Lv J, Gao Y, Zhong J, Niu J, Sun B. Interferon alpha (IFNalpha)-induced TRIM22 interrupts HCV replication by ubiquitinating NS5A. Cell Mol Immunol. 2016;13(1):94–102.
Liang Y, Zhang G, Li Q, Han L, Hu X, Guo Y, Tao W, Zhao X, Guo M, Gan T, Tong Y, Xu Y, Zhou Z, Ding Q, Wei W, Zhong J. TRIM26 is a critical host factor for HCV replication and contributes to host tropism. Sci Adv. 2021;7(2):1–8.
Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci. 2021;22(11):5801.
Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Can Res. 2017;77(21):e108–10.
Park HH, Kim HR, Park SY, Hwang SM, Hong SM, Park S, Kang HC, Morgan MJ, Cha JH, Lee D, Roe JS, Kim YS. RIPK3 activation induces TRIM28 derepression in cancer cells and enhances the anti-tumor microenvironment. Mol Cancer. 2021;20(1):107.
Lin J, Guo D, Liu H, Zhou W, Wang C, Muller I, Kossenkov AV, Drapkin R, Bitler BG, Helin K, Zhang R. The SETDB1-TRIM28 complex suppresses antitumor immunity. Cancer Immunol Res. 2021;9(12):1413–24.
Liang J, Wang L, Wang C, Shen J, Su B, Marisetty AL, Fang D, Kassab C, Jeong KJ, Zhao W, Lu Y, Jain AK, Zhou Z, Liang H, Sun SC, Lu C, Xu ZX, Yu Q, Shao S, Chen X, Gao M, Claret FX, Ding Z, Chen J, Chen P, Barton MC, Peng G, Mills GB, Heimberger AB. Verteporfin inhibits PD-L1 through autophagy and the STAT1-IRF1-TRIM28 signaling axis. Exerting Antitumor Efficacy Cancer Immunol Res. 2020;8(7):952–65.
Ma X, Ma X, Zhu L, Zhao Y, Chen M, Li T, Lin Y, Ma D, Sun C, Han L. The E3 ubiquitin ligase MG53 inhibits hepatocellular carcinoma by targeting RAC1 signaling. Oncogenesis. 2022;11(1):40.
Fan W, Liu X, Ren D. TRIM62 silencing represses the proliferation and invasion and increases the chemosensitivity of hepatocellular carcinoma cells by affecting the NF-kappaB pathway. Toxicol Appl Pharmacol. 2022;445: 116035.
Zheng T, Yin D, Lu Z, Wang J, Li Y, Chen X, Liang Y, Song X, Qi S, Sun B, Xie C, Meng X, Pan S, Liu J, Jiang H, Liu L. Nutlin-3 overcomes arsenic trioxide resistance and tumor metastasis mediated by mutant p53 in hepatocellular carcinoma. Mol Cancer. 2014;13:133.
Cui L, Zhang S, Zhang W, Hu Z, Cao Z, Li T. Arsenic trioxide and promyelocytic leukemia protein-adenovirus synergistically inhibit in vitro and in vivo growth of a hepatoma cell line. Oncol Res. 2010;18(7):305–14.
Tan CRC, Abdul-Majeed S, Cael B, Barta SK. Clinical pharmacokinetics and pharmacodynamics of bortezomib. Clin Pharmacokinet. 2019;58(2):157–68.
Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14(7):417–33.
Kim GP, Mahoney MR, Szydlo D, Mok TS, Marshke R, Holen K, Picus J, Boyer M, Pitot HC, Rubin J, Philip PA, Nowak A, Wright JJ, Erlichman C. An international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30(1):387–94.
Chen YJ, Wu H, Shen XZ. The ubiquitin-proteasome system and its potential application in hepatocellular carcinoma therapy. Cancer Lett. 2016;379(2):245–52.
Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–20.
Burslem GM, Crews CM. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell. 2020;181(1):102–14.
Dale B, Cheng M, Park KS, Kaniskan HU, Xiong Y, Jin J. Advancing targeted protein degradation for cancer therapy. Nat Rev Cancer. 2021;21(10):638–54.
Qi SM, Dong J, Xu ZY, Cheng XD, Zhang WD, Qin JJ. PROTAC: an effective targeted protein degradation strategy for cancer therapy. Front Pharmacol. 2021;12: 692574.
Gechijian LN, Buckley DL, Lawlor MA, Reyes JM, Paulk J, Ott CJ, Winter GE, Erb MA, Scott TG, Xu M, Seo HS, Dhe-Paganon S, Kwiatkowski NP, Perry JA, Qi J, Gray NS, Bradner JE. Functional TRIM24 degrader via conjugation of ineffectual bromodomain and VHL ligands. Nat Chem Biol. 2018;14(4):405–12.
Shah VV, Duncan AD, Jiang S, Stratton SA, Allton KL, Yam C, Jain A, Krause PM, Lu Y, Cai S, Tu Y, Zhou X, Zhang X, Jiang Y, Carroll CL, Kang Z, Liu B, Shen J, Gagea M, Manu SM, Huo L, Gilcrease M, Powell RT, Guo L, Stephan C, Davies PJ, Parker-Thornburg J, Lozano G, Behringer RR, Piwnica-Worms H, Chang JT, Moulder SL, Barton MC. Mammary-specific expression of Trim24 establishes a mouse model of human metaplastic breast cancer. Nat Commun. 2021;12(1):5389.
Mahon C, Krogan NJ, Craik CS, Pick E. Cullin E3 ligases and their rewiring by viral factors. Biomolecules. 2014;4(4):897–930.
Chiang C, Pauli EK, Biryukov J, Feister KF, Meng M, White EA, Munger K, Howley PM, Meyers C, Gack MU. The human papillomavirus E6 oncoprotein targets USP15 and TRIM25 to suppress RIG-I-mediated innate immune signaling. J Virol. 2018;92(6):19.
Sewatanon J, Ling PD. Murine gammaherpesvirus 68 ORF75c contains ubiquitin E3 ligase activity and requires PML SUMOylation but not other known cellular PML regulators, CK2 and E6AP, to mediate PML degradation. Virology. 2013;440(2):140–9.
UniProt C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49(D1):D480–9.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Nature Science Foundation of China (Grant Nos. 81874065 and 81874149), National Basic Research Program of China (Grant No. 2020YFA0710700), Tongji Hospital (HUST) Fundation for Excellent Young Scientist (Grant No. 2020YQ05) and the first level of the public health youth top talent project of Hubei province.
Author information
Authors and Affiliations
Contributions
KL collected the related papers, generates the data, prepared figures, and was a major contributor in writing the manuscript. YP, ZH, and HL revised the manuscript. BZ and ZD initiated the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Oncoprint plot of all somatic mutations of TRIM proteins in TCGA-LIHC using cbioportal database.
Additional file 2: Table S1.
Differential gene expression under different mutation status in HCC through TIMER2.0. The logFC with statistical significance (p<0.05) are colored in the table. Table S2. Cox analysis for all TRIM proteins based on TCGA-LIHC patients with OS more than a month. Table S3. Intracellular location of TRIM proteins from uniprot.
Additional file 3: Fig. S2.
A univariate cox analysis of every 75 TRIM proteins based on TCGA-LIHC dataset with p < 0.05.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, K., Pan, Y., Huang, Z. et al. TRIM proteins in hepatocellular carcinoma. J Biomed Sci 29, 69 (2022). https://doi.org/10.1186/s12929-022-00854-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-022-00854-7