Abstract
Background
Knee osteoarthritis (OA) is one of the leading causes of disability within the adult population. Current treatment options for OA of the knee include intra-articular (IA) hyaluronic acid (HA), a molecule found intrinsically within the knee joint that provides viscoelastic properties to the synovial fluid. A variety of mechanisms in which HA is thought to combat knee OA are reported in the current basic literature.
Methods
We conducted a comprehensive literature search to identify currently available primary non-clinical basic science articles focussing on the mechanism of action of IA-HA treatment. Included articles were assessed and categorized based on the mechanism of action described within them. The key findings and conclusions from each included article were obtained and analyzed in aggregate with studies of the same categorical assignment.
Results
Chondroprotection was the most frequent mechanism reported within the included articles, followed by proteoglycan and glycosaminoglycan synthesis, anti-inflammatory, mechanical, subchondral, and analgesic actions. HA-cluster of differentiation 44 (CD44) receptor binding was the most frequently reported biological cause of the mechanisms presented. High molecular weight HA was seen to be superior to lower molecular weight HA products. HA derived through a biological fermentation process is also described as having favorable safety outcomes over avian-derived HA products.
Conclusions
The non-clinical basic science literature provides evidence for numerous mechanisms in which HA acts on joint structures and function. These actions provide support for the purported clinical benefit of IA-HA in OA of the knee. Future research should not only focus on the pain relief provided by IA-HA treatment, but the disease modification properties that this treatment modality possesses as well.
Similar content being viewed by others
Background
Although osteoarthritis (OA) of the knee is most often a slowly progressive joint disorder, it is one of the leading causes of disability of the adult population [1]. Knee OA, a disease of the entire joint, is characterized by joint pain, cartilage degeneration, and an increase in disability [2]. The progressive nature of OA leads to decreased knee function, affecting an individual’s ability to perform daily activities [3]. Knee OA also negatively impacts socioeconomic factors, as the associated disability often leads to impaired work performance and early retirement [4].
Since there is no established disease modifying agent for OA, there are many options for the treatment of knee OA. Among the pharmacologic therapies, non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular (IA) corticosteroid injections are most commonly prescribed [5]. These options have inherent limitations, as NSAIDs have potentially serious adverse events associated with their use [6], and IA corticosteroid injections often provide a relatively short period of effective relief [7]. Although corticosteroid injection generally has a positive safety profile, it has been shown to cause a transient increase in blood glucose, which may be a concern for diabetic patients [8]. IA injection of hyaluronic acid (HA) is another treatment option for knee OA pain. HA is nearly ubiquitous in the body, and is a molecule found intrinsically within the knee joint where it provides viscoelastic properties to synovial fluid [9]. As OA progresses, natural HA concentration and the distribution of HA within the joint shifts towards lower ranges of HA molecular weight, leading to a degradation of the mechanical/viscoelastic properties of the endogenous synovial fluid [2, 10, 11]. Lower ranges of molecular weight distributions have also been shown to be strongly correlated to pain [11]. IA-HA administration has aimed to restore this decline in HA concentration and the average molecular weight distribution within the OA knee [9].
IA-HA has been proposed to have many therapeutic mechanisms of action in the OA knee, including shock absorption, joint lubrication, anti-inflammatory effects, chondroprotection, proteoglycan synthesis, and cartilage matrix alterations [2]. The correlation between these various effects has created a better understanding of how IA-HA treatment could provide therapeutic effects for patients with knee OA [12]. There is also evidence suggesting distinct mechanism of action differences between HA products of varying molecular weight. That is, higher molecular weight (HMW) HA has been reported to provide greater anti-inflammatory and proteoglycan synthesis effects, as well as joint lubrication and viscoelasticity maintenance [9, 13]. There also appears to be evidence of variable safety profiles between HA derived through a biological fermentation process (Bio-HA) and avian-derived HA (AD-HA), as AD-HA has the potential for local IA reactions [14, 15].
We aim to summarize the mechanisms of action for IA-HA treatment of knee OA described in the current literature in order to determine the validity of the above mechanisms of action. We will systematically assess and outline the defined mechanisms in which HA may provide a therapeutic benefit, while analyzing reported distinction between product characteristic-dependent effects of IA-HA treatment.
Methods
Literature search
We conducted a comprehensive literature search using the MEDLINE, EMBASE, and PubMed databases (Table 1). The search was conducted on May 4th, 2014. The inclusion criteria followed throughout the screening process were as follows: 1) Articles describing the mechanism of HA treatment for OA, 2) Articles focused on OA of the knee, and 3) Primary non-clinical basic science articles focussing on “viscosupplementation”/HA treatment. Articles that were published before 1990 or were not published in English were excluded from the study. If multiple studies outlining similar results were published by the same author, only the most recent study was accepted for inclusion.
Data abstraction
Included articles were assessed and categorized based on the mechanism of action described within them. Single articles were included in multiple categories if they analyzed more than one mechanism of HA action within their study design. The mechanism categories utilized in the data abstraction process were: chondroprotection, alterations in proteoglycan and/or glycosaminoglycan (GAG) synthesis, anti-inflammatory effects, mechanical modifications, alterations in subchondral bone, and analgeisic effects.
Data analysis
The key findings and conclusions from each included article were obtained and analyzed in aggregate with studies of the same assigned category. The results presented have been derived through interpretation of common and consistent mechanism of action presentations within each aforementioned category.
Results
Search strategy
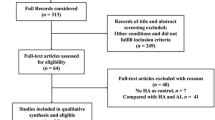
The literature search identified 2,782 potential articles and, of these, 91 articles met the inclusion criteria (Fig. 1). Thirteen additional articles were retrieved by a content expert’s hand search of the literature. This resulted in a total of 104 articles included. The conclusions and key findings of each article were identified within the aforementioned mechanism categories (Table 2).
Chondroprotection
Sixty-seven of the included articles described chondroprotective effects of IA-HA treatment [9, 12, 16–80]. IA-HA has been shown to reduce chondrocyte apoptosis, while increasing chondrocyte proliferation [19, 20]. There are multiple observed effects of IA-HA treatment that produce chondroprotection, many of which are results of HA binding to cluster of differentiation 44 (CD44) receptors. HA binding to CD44 inhibits interleukin (IL)-1β expression, leading to a decline in matrix metalloproteinase (MMP) -1, 2, 3, 9, and 13 productions [32, 34]. This binding to CD44 has been shown to be of greater effect for higher MW HA products [21]. HA also binds to the receptor for hyaluronan mediated motility (RHAMM), which is thought to aid in chondroprotection in addition to CD44 binding [34]. The inhibition of IL-1β expression through CD44 binding is carried out through induction of mitogen-activated protein kinase phosphatase (MKP) -1: a negative regulator of IL-1β [43]. This inhibition of various MMPs impedes catabolic enzyme activity within the joint cartilage [59]. HMW HA was shown to have a greater effect in the inhibitory action of MMP production [21], although these results are unclear, as another study has demonstrated the favourable MMP inhibitory effect of lower molecular weight (LMW) products [72].
Chondrocyte apoptotic events are further decreased by HA-CD44 binding through the reduction of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) expression [38]. These peptidases are involved in the cleavage of important synovial components, including aggrecan, versican, and brevican [81, 82]. Various ADAMTS expression has been shown to decrease as a result of HA-CD44 binding, providing an additional mode of chondroprotection for IA-HA treatment [36, 38, 43]. The production of reactive oxygen species (ROS), such as nitric oxide (NO), results in degeneration of cartilage through increased chondrocyte apoptosis [33]. IA-HA treatment demonstrated a reduction in IL-1β-induced oxidative stress, through inhibition of NO production within the synovium [48, 57]. Additional results of CD44-HA binding resulting in chondroprotective effects mentioned in the current literature include reduction of prostaglandin E2 (PGE2) synthesis [42, 46, 61], and increased heat shock protein 70 (Hsp70) overexpression [25, 70]. These effects similarly provide therapeutic benefit through reduction of chondrocyte apoptosis. It is important to note that HMW HA products demonstrated greater inhibition of PGE2 expression than LMW comparators in a comparative study, resulting in greater chondroprotective effects [61].
Proteoglycan and glycosaminoglycan synthesis
Twenty-two of the identified studies reported on the enhanced proteoglycan and glycosaminoglycan synthesis related to IA-HA treatment [27, 42, 44, 48, 62, 69, 83–98]. As OA progresses, intrinsic proteoglycan and GAG concentrations decline within the cartilage. Results demonstrated that IA-HA treatment stimulated proteoglycan synthesis, delaying the progression of OA [69, 88]. Aggrecan is the primary proteoglycan within articular cartilage, and IA-HA treatment is shown to both suppress aggrecan degradation, as well as promote intrinsic aggrecan development [62, 93]. IA-HA treatment is shown to mobilize newly synthesized proteoglycan to the outer chondrocyte matrix, potentially providing protection from degradation. Extrinsic HA promoted movement of newly synthesized proteoglycan from the cell-associated matrix to the further-removed matrix in an alginate gel model, which suggests that IA-HA could provide therapeutic relief of OA by strengthening the interterritorial cartilage matrix [92]. A marker of proteoglycan synthesis, sulphate (35SO4 ), is seen to be increasingly incorporated within chondrocytes after HA introduction [87]. The biological pathway in which HA alters aggrecan levels is shown to be through CD44 and intercellular adhesion molecule (ICAM)-1 binding effects [62]. HMW HA was shown by one study to provide a greater effect of proteoglycan synthesis than LMW HA through stimulation of the insulin-like growth factor (IGF)-1 pathway [89]. IA-HA treatment is also shown to increase endogenous GAG production. IA-HA treatment not only supplemented the synovium with HA, it promoted intrinsic production of HA [27].
Anti-inflammatory
Twenty-one of the identified studies reported on the anti-inflammatory effects of IA-HA treatment [13, 20, 21, 25, 27, 52, 54, 55, 68, 72, 96, 97, 99–107]. IL-1β is known to demonstrate pro-inflammatory effects, and the aforementioned suppression of IL-1β expression by HA provides anti-inflammatory effects [52]. IL-1β is a key mediator in the anti-inflammatory effects of HA, and is regulated through HA-CD44 binding [59, 104]. IL-1β suppression results in a down-regulation of MMPs as previously mentioned, which also aids in the anti-inflammatory effect of HA [52]. Further suppression of pro-inflammatory mediators IL-8, IL-6, PGE2, and tumor necrosis factor (TNFα) provides anti-inflammatory effects of IA-HA treatment [13, 21]. The relation between Toll-Like Receptors (TLR) and an inflammatory response is demonstrated as HA degradation products induced an inflammatory response through CD44 and TLR interaction. This pro-inflammatory response from HA degradation product binding to TLR and CD44 receptors results in increased Nf-kB, IL-1β, TNFα, IL-6, and IL-33 production [105]. HMW HA has been demonstrated to suppress numerous inflammatory mediators through TLR 2 and 4 binding, including TNF-α, IL-1-β, IL-17, MMP-13 and inducible nitrogen oxide synthase (iNOS) [106]. A direct correlation between molecular weight and anti-inflammatory effects has demonstrated larger effects of PGE2 and IL-6 inhibition for HMW HA treatment [13]. IL-6 is a pro-inflammatory cytokine, regulated by nuclear factor kappa-light-chain-enhancer of activated B cells (Nf-кB). HA binding to ICAM-1 down regulates Nf-kB, which in turn decreases the production of IL-6 [21, 104]. HMW HA down regulation of TNFα, IL-1B and IL-8 is an additional contributory factor to the anti-inflammatory effects provided by HMW HA [72, 97].
Mechanical
Ten of the included studies described mechanical effects of HA in the treatment of knee OA [41, 49, 86, 108–114]. The viscous nature of HA treatment is shown to lubricate the joint capsule, preventing degeneration through decreased friction [86]. HA further protects the joint capsule through beneficial shock absorption effects. HA provides cushioning to absorb pressure and vibration within the joint that otherwise may lead to chondrocyte degradation [41]. Osteoarthritic knees are reported to have higher friction within the joint space than healthy knees, which is counteracted by the joint lubrication capabilities that HA possesses [110]. HMW HA has been demonstrated to provide a greater effect of friction reduction due to its viscous properties. The reduction of friction within the joint can provide therapeutic effects, as the cartilage is protected from mechanical degradation [111].
Subchondral bone
Eight of the included studies outlined the effects that IA-HA has on the subchondral bone [23, 31, 32, 45, 115–118]. It previously has been shown that interaction between subchondral bone osteoblasts and articular cartilage chondrocytes in osteoarthritic joints alters ADAMTS-4/5 and MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13 expression and regulation, mediated by mitogen-activated protein kinase (MAPK) and extracellular signal regulated kinase 1 and 2 (ERK 1/2) signalling pathways [118]. IA-HA also affects the subchondral bone through suppression of MMP-13 and IL-6 via CD44 binding, which potentially prevents abnormal osseous tissue metabolism [116]. The suppression of MMP-13 expression by IA-HA has been suggested to be a critical factor in the effect on OA subchondral bone [117]. The effect that IA-HA has on MMPs, specifically MMP-13, through CD44 binding has been suggested to inhibit the effects of OA within the subchondral bone in numerous non-clinical basic science investigations [32, 45, 116–118]. HA effectively changes subchondral bone density and thickness through trabecular structure alterations, resulting in greater subchondral bone compliancy. This ultimately is shown to reduce the stress put on cartilage during impact loading [23]. An indication of IA-HA stimulation of cartilage/bone interface type II collagen turnover is the increase of urine carboxy-terminal collagen crosslinks (CTX)-II levels observed following IA-HA treatment, which demonstrated improvement in the osteoarthritic knee [31]. Ultimately, HA appeared to limit subchondral bone changes that are characteristic of early OA [115].
Analgesic
Six of the included articles described the analgesic effects of IA-HA treatment [76, 80, 119–122]. A single injection of HA demonstrated a significant decrease in pain-associated behaviour within a murine model [119]. One study suggested that HA did not directly bind to bradykinin receptors, but provided analgesic effects through interaction with HA receptors and/or free nerve endings within the joint tissue [121]. HA analgesic effects have been shown to occur at mechanosensitive stretch-activated ion channels, where channel activity is significantly decreased upon HA binding [122]. HMW HA was shown to decrease mechanical sensitivity of stretch-activated ion channels, which effectively blocked the pain response. LMW HA was seen to be less effective in blocking this response [122]. HA reduces the action of joint nociceptors, which provides pain reduction within the joint. Sensitized nociceptive terminals within the joint tissue are affected by HA concentration, reducing the pain response exhibited by these terminals [120].
Discussion
Our review of the existing literature provides a general consensus that IA-HA for knee OA has beneficial effects through several mechanisms of action; however, the predominant mechanism in which therapeutic effect is provided is not clearly understood [2]. In perspective, it is not clear which of these mechanisms are clinically relevant, as it is appreciated that beneficial mechanisms of action are not necessarily transferrable to benefit in the clinical setting. At this time, it is presumed that the clinical benefit of IA-HA in knee OA is due to several concurrent mechanisms of action, instead of any one single specific mechanism of action.
The majority of exogenous HA remains in the joint for a few days; however, the clinical therapeutic effects of HA treatment may be seen for up to 6 months, or more. This may suggest that IA-HA contains disease modifying properties, and does not act solely by restoring viscoelastic properties to the synovial fluid [123]. HA injections may stimulate endogenous production of additional HA by human synoviocytes, aiding in the normalization of HA distribution within the synovial fluid [124]. A large number of reports describe CD44 binding as a primary mode in which HA provides action against knee OA in non-clinical basic science studies. CD44-mediated effects of IA-HA are shown to contribute to the potential chondroprotection, proteoglycan/glycosaminoglycan synthesis, anti-inflammatory, and subchondral mechanisms. This binding is shown to have a variety of effects on numerous signalling pathways, all of which demonstrate some sort of intervention in the progression of OA [13, 21, 32, 36, 38, 42, 43, 46, 59, 61, 104, 116]. The suppression of IL-1β and IL-6 and subsequent effects of this suppression have been suggested to be a key factor in the therapeutic mechanism provided by HA-CD44 binding [123]. It is evident that HA-CD44 binding is a major component in the mechanism in which HA provides therapeutic effect; however, there are additional mechanisms that provide alternate pathways for the effectiveness of HA treatment in OA knees, including ICAM binding, mechanical improvements attributed to shock absorption and lubrication, an increase in cartilage/bone interface type II collagen turnover, as well as analgesic effects through interaction with nerve endings and joint nociceptors [31, 41, 86, 97, 121, 122]. HA binding to the RHAMM receptor promotes wound repair, activates pro-migration and invasion functions, regulates cellular responses to growth factors, and plays a role in fibroblast migration and motility [125–127]. These results of HA-RHAMM binding are potential factors involved in the disease modification effects of HA treatment for OA.
There is evidence which demonstrates that certain intrinsic properties of particular IA-HA products may provide beneficial results in comparison to other IA-HA products. The most recognized of these intrinsic properties is molecular weight. Contrary to a previous basic science review by Ghosh et al., which suggested a potential benefit of LMW HA in providing rheological property restoration over HMW HAs [128], the evidence within the current review has demonstrated advantageous results for HMW HA treatments. The current review supports the view that HMW HA provides superior chondroprotective, proteoglycan and glycosaminoglycan synthesis, anti-inflammatory, mechanical, and analgesic mechanisms of action [61, 89, 97, 111, 122]. A study by Huang et al. demonstrated superior anti-inflammatory effects of HMW HA but superior chondroprotective effects of LMW HA; however, these results regarding chondroprotection are unclear due to lack of additional evidence within the knee OA basic science literature [72]. An increased production of inflammatory cytokines and chemokines, recruitment of inflammatory mediators, and blood vessel formation have been shown to be a response to LMW HA below 500 kDa. While the average MW of available HA products on the market vary greatly, it should be noted that, to our knowledge, all currently available products worldwide have a molecular weight >500 kDa [129, 130]. An analysis of HA-CD44 interaction demonstrated that HA size has direct impact on the affinity in which HA binds to the CD44 receptor [131]. These results demonstrate the capacity of HMW HA to treat the progression of knee OA through CD44 binding of HA. These basic science findings are consistent with systematic reviews of clinical trials and comparative studies which have demonstrated that HMW HA provides greater therapeutic benefit than LMW HA in the treatment of knee OA [6, 132], although the current literature does not provide consensus regarding the clinical efficacy difference between low and high molecular weight HA [133].
Traditionally, HA products had been derived from avian sources; however, some available products are produced by biological fermentation. This process avoids the presence of avian-derived molecules which are suggested to be a potential cause of adverse local reactions [134]. There is a lack of thorough reporting regarding the potential of Bio-HA over AD-HA. One study has suggested AD-HA injection sites may be the cause of synovitis in their patient group, yet the exact pathological agent is unknown [135]. Results from a second study also outline the potential for hylan AD-HA to cause a foreign body giant cell type granulomatous reaction [136]. Research has demonstrated that the flare-ups associated with hylan injection may be correlated to the accumulation of hylan or its breakdown products, as injection site flare ups typically do not occur upon first injection [14]. Avian derived proteins have been shown to be the cause of injection site flare up, as antibodies to chicken serum protein were present in patients who demonstrated injection site adverse reaction after being treated with AD-HA [15]. There is some high-quality clinical evidence that Bio-HA has a significantly smaller incidence of injection site adverse events than AD-HA [134]; however, this is not thoroughly investigated within the current literature. More comprehensive investigation of the difference in incidence of injection site adverse events between Bio-HA and AD-HA from both a basic science and clinical perspective is needed.
This review has methodological strength in its systematic and thorough search of available basic science evidence within multiple databases. The current report also demonstrates rigor in its presentation of multiple mechanisms in which HA acts, providing evidence on all mechanisms whether comprehensively or infrequently reported within the current literature. Limitations of the current study arise due to the subjective classification of included article mechanism of action key conclusions. Included articles may briefly mention alternate mechanisms of action, but were not classified into the corresponding category because the mentioned alternate mechanism was not a key result or conclusion of the study. Future research should analyze the relationship between the various mechanisms presented in this report, and clarify the way in which these mechanisms overlap and may work together to alleviate symptoms of knee OA. Future research should also aim to recognize differences between mechanisms exhibited by high and lower molecular weight products, as well as analyze the safety profile differences between Bio-HA and AD-HA.
Conclusions
The non-clinical basic science literature provides evidence for numerous mechanisms in which IA-HA may provide clinical benefit in knee OA. Chondroprotection is the most frequently reported mechanism, with HA-CD44 binding being the most frequently reported source of these effects. IA-HA is also reported to provide proteoglycan and glycosaminoglycan synthesis, anti-inflammatory, mechanical, subchondral, and analgesic effects. There is evidence of favorable results for HMW HA treatments in comparison to LMW HA. Bio HA is also demonstrated to provide an advantageous safety profile over AD-HA, as reports demonstrate the association between injection site flare ups and avian-derived proteins. There are a variety of reported mechanisms in which IA-HA is demonstrated to treat knee OA, as well as numerous product characteristics that impact the results of IA-HA treatment. A thorough understanding of the variety of mechanisms in which IA-HA provides beneficial effects within the OA knee, as well as the characteristic-specific effects of various IA-HA products, is required to recognize the applicability and appropriateness of IA-HA treatment for knee OA. Future research should not only focus on the pain relief provided by IA-HA treatment, but the disease modification properties that this treatment modality may possess as well.
Abbreviations
- ADAMTS:
-
A disintegrin and metalloproteinase with thrombospondin motifs
- AD-HA:
-
Avian-derived hyaluronic acid
- Bio-HA:
-
Biologically-derived hyaluronic acid
- CD:
-
Cluster of differentiation
- CTX:
-
Carboxy-terminal collagen crosslinks
- ERK 1/2:
-
Extracellular signal regulated kinase 1 and 2
- GAG:
-
Glycosaminoglycan
- HA:
-
Hyaluronic acid
- HMW:
-
Higher molecular weight
- Hsp70:
-
Heat shock protein 70
- IA:
-
Intra-articular
- ICAM:
-
Intercellular adhesion molecule
- IGF:
-
Insulin-like growth factor
- IL:
-
Interleukin
- iNOS:
-
Inducible nitrogen oxideLMW, lower molecular weight
- MAPK:
-
Mitogen-activated protein kinase
- MKP:
-
Protein kinase phosphate
- MMP:
-
Metalloproteinase
- Nf-кB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO:
-
Nitric oxide
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- PGE2:
-
Prostaglandin E2
- RHAMM:
-
Receptor for hyaluronan mediated motility
- ROS:
-
Reactive oxygen species
- TLR:
-
Toll-like receptor
References
Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13(6):740–53.
Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Therapy. 2003;5(2):54.
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2, CD005321.
Trigkilidas D, Anand A. The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. Ann R Coll Surg Engl. 2013;95(8):545–51.
Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26(4):257–68.
Rutjes A, Juni P, da Costa B, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180–91.
Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–11.
Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28(7):749–56.
Elmorsy S, Funakoshi T, Sasazawa F, Todoh M, Tadano S, Iwasaki N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthritis Cartilage. 2014;22(1):121–7.
Kosinska MK, Ludwig TE, Liebisch G, Zhang R, Siebert HC, Wilhelm J, et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One. 2015;10(5), e0125192.
Band PA, Heeter J, Wisniewski HG, Liublinska V, Pattanayak CW, Karia RJ, et al. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthritis Cartilage. 2015;23(1):70–6.
Diaz-Gallego L, Prieto JG, Coronel P, Gamazo LE, Gimeno M, Alvarez AI. Apoptosis and nitric oxide in an experimental model of osteoarthritis in rabbit after hyaluronic acid treatment. J Orthop Res. 2005;23(6):1370–6.
Lajeunesse D, Delalandre A, Martel-Pelletier J, Pelletier J-P. Hyaluronic acid reverses the abnormal synthetic activity of human osteoarthritic subchondral bone osteoblasts. Bone. 2003;33(4):703–10.
Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol Dec. 2002;29(12):2611–4.
Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan viscosupplementation: diagnosis and treatment. Clin Orthop Relat Res Feb. 2004;419:130–7.
Ando A, Hagiwara Y, Chimoto E, Hatori K, Onoda Y, Itoi E. Intra-articular injection of hyaluronan diminishes loss of chondrocytes in a rat immobilized-knee model. Tohoku J Exp Med. 2008;215(4):321–31.
Ariyoshi W, Okinaga T, Knudson CB, Knudson W, Nishihara T. High molecular weight hyaluronic acid regulates osteoclast formation by inhibiting receptor activator of NF-kappaB ligand through Rho kinase. Osteoarthritis Cartilage. 2014;22(1):111–20.
Asari A, Miyauchi S, Matsuzaka S, Itoh T, Uchiyama Y. Hyaluronate on heat shock protein and synovial cells in a canine model of osteoarthritis. Osteoarthritis Cartilage. 1996;4(3):213–5.
Brun P, Panfilo S, Daga Gordini D, Cortivo R, Abatangelo G. The effect of hyaluronan on CD44-mediated survival of normal and hydroxyl radical-damaged chondrocytes. Osteoarthritis Cartilage. 2003;11(3):208–16.
Brun P, Zavan B, Vindigni V, Schiavinato A, Pozzuoli A, Iacobellis C, et al. In vitro response of osteoarthritic chondrocytes and fibroblast-like synoviocytes to a 500-730 kDa hyaluronan amide derivative. J Biomed Mater Res B Appl Biomater. 2012;100(8):2073–81.
Chang CC, Hsieh MS, Liao ST, Chen YH, Cheng CW, Huang PT, et al. Hyaluronan regulates PPARgamma and inflammatory responses in IL-1beta-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr Polym. 2012;90(2):1168–75.
Creamer P, Sharif M, George E, Meadows K, Cushnaghan J, Shinmei M, et al. Intra-articular hyaluronic acid in osteoarthritis of the knee: an investigation into mechanisms of action. Osteoarthritis Cartilage. 1994;2(2):133–40.
Ding M, Christian Danielsen C, Hvid I. Effects of hyaluronan on three-dimensional microarchitecture of subchondral bone tissues in guinea pig primary osteoarthrosis. Bone. 2005;36(3):489–501.
Ehlers EM, Behrens P, Wunsch L, Kuhnel W, Russlies M. Effects of hyaluronic acid on the morphology and proliferation of human chondrocytes in primary cell culture. Ann Anat. 2001;183(1):13–7.
Galois L, Etienne S, Henrionnet C, Scala-Bertola J, Grossin L, Mainard D, et al. Ambivalent properties of hyaluronate and hylan during post-traumatic OA in the rat knee. Biomed Mater Eng. 2012;22(4):235–42.
Gonzalez-Fuentes AM, Green DM, Rossen RD, Ng B. Intra-articular hyaluronic acid increases cartilage breakdown biomarker in patients with knee osteoarthritis. Clin Rheumatol. 2010;29(6):619–24.
Greenberg DD, Stoker A, Kane S, Cockrell M, Cook JL. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthritis Cartilage. 2006;14(8):814–22.
Grishko V, Xu M, Ho R, Mates A, Watson S, Kim JT, et al. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem. 2009;284(14):9132–9.
Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthritis Cartilage. 2009;17(11):1513–8.
Homandberg GA, Hui F, Wen C, Kuettner KE, Williams JM. Hyaluronic acid suppresses fibronectin fragment mediated cartilage chondrolysis: I. In vitro. Osteoarthritis Cartilage. 1997;5(5):309–19.
Ishijima M, Nakamura T, Shimizu K, Hayashi K, Kikuchi H, Soen S, et al. Different changes in the biomarker CTX-II following intra-articular injection of high molecular weight hyaluronic acid and oral non-steroidal anti-inflammatory drugs for patients with knee osteoarthritis: a multi-center randomized controlled study. Osteoarthr Cartil. 2013;21:S292.
Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004;50(2):516–25.
Kalaci A, Yilmaz HR, Aslan B, Sogut S, Yanat AN, Uz E. Effects of hyaluronan on nitric oxide levels and superoxide dismutase activities in synovial fluid in knee osteoarthritis. Clin Rheumatol. 2007;26(8):1306–11.
Karna E, Miltyk W, Surazynski A, Palka JA. Protective effect of hyaluronic acid on interleukin-1-induced deregulation of beta1-integrin and insulin-like growth factor-I receptor signaling and collagen biosynthesis in cultured human chondrocytes. Mol Cell Biochem. 2008;308(1-2):57–64.
Kobayashi K, Amiel M, Harwood FL, Healey RM, Sonoda M, Moriya H, et al. The long-term effects of hyaluronan during development of osteoarthritis following partial meniscectomy in a rabbit model. Osteoarthritis Cartilage. 2000;8(5):359–65.
Koga H. Effects of hyaluronic acid on arthritic articular cartilage. Connect Tissue Res. 2012;53(1):48–93.
Lazaro J, Granado P, del Sol G, Medina A, Gallego L, Sandoval D, et al. The role of different hyaluronic acids in the articular cartilage of rabbit. Open Orthopaedics J. 2010;4:44–7.
Li J, Gorski DJ, Anemaet W, Velasco J, Takeuchi J, Sandy JD, et al. Hyaluronan injection in murine osteoarthritis prevents TGFbeta 1-induced synovial neovascularization and fibrosis and maintains articular cartilage integrity by a CD44-dependent mechanism. Arthritis Res Ther. 2012;14(3):R151.
Li P, Raitcheva D, Hawes M, Moran N, Yu X, Wang F, et al. Hylan G-F 20 maintains cartilage integrity and decreases osteophyte formation in osteoarthritis through both anabolic and anti-catabolic mechanisms. Osteoarthritis Cartilage. 2012;20(11):1336–46.
Lisignoli G, Grassi F, Zini N, Toneguzzi S, Piacentini A, Guidolin D, et al. Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) invovement. Arthritis Rheum. 2001;44(8):1800–7.
Lu HT, Sheu MT, Lin YF, Lan J, Chin YP, Hsieh MS, et al. Injectable hyaluronic-acid-doxycycline hydrogel therapy in experimental rabbit osteoarthritis. BMC Vet Res. 2013;9:68.
Maneiro E, de Andres MC, Fernandez-Sueiro JL, Galdo F, Blanco FJ. The biological action of hyaluronan on human osteoartritic articular chondrocytes: the importance of molecular weight. Clin Exp Rheumatol. 2004;22(3):307–12.
Mihara M, Hashizume M. The effect of high molecular hyaluronic acid on the induction of matrix degradation enzymes By IL-6, IL-1β and TNF-α. Osteoarthr Cartil. 2012;20:S134–5.
Miki Y, Teramura T, Tomiyama T, Onodera Y, Matsuoka T, Fukuda K, et al. Hyaluronan reversed proteoglycan synthesis inhibited by mechanical stress: possible involvement of antioxidant effect. Inflamm Res. 2010;59(6):471–7.
Mladenovic Z, Saurel AS, Berenbaum F, Jacques C. Potential role of hyaluronic acid on bone in osteoarthritis: matrix metalloproteinases, aggrecanases, and RANKL expression are partially prevented by hyaluronic acid in interleukin 1-stimulated osteoblasts. J Rheumatol. 2014;41(5):945–54.
Mongkhon JM, Thach M, Shi Q, Fernandes JC, Fahmi H, Benderdour M. Sorbitol-modified hyaluronic acid reduces oxidative stress, apoptosis and mediators of inflammation and catabolism in human osteoarthritic chondrocytes. Inflamm Res. 2014;63(8):691–701.
Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharide-induced activation of transcription factors in bovine articular chondrocytes. Arthritis Rheum. 2005;52(3):800–9.
Peng H, Zhou JL, Liu SQ, Hu QJ, Ming JH, Qiu B. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res. 2010;59(7):519–30.
Plaas A, Li J, Riesco J, Das R, Sandy JD, Harrison A. Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis. Arthritis Res Ther. 2011;13(2):R46.
Qiu B, Liu SQ, Peng H. Influence of sodium hyaluronate on iNOS expression in synovium and NO content in synovial fluid of rabbits with traumatic osteoarthritis. Chin J Traumatol. 2008;11(5):293–6.
Sakakibara Y, Miura T, Iwata H, Kikuchi T, Yamaguchi T, Yoshimi T, et al. Effect of high-molecular-weight sodium hyaluronate on immobilized rabbit knee. Clin Orthop Relat Res. 1994;299:282–92.
Sasaki A, Sasaki K, Konttinen YT, Santavirta S, Takahara M, Takei H, et al. Hyaluronate inhibits the interleukin-1beta-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med. 2004;204(2):99–107.
Shimizu C, Yoshioka M, Coutts RD, Harwood FL, Kubo T, Hirasawa Y, et al. Long-term effects of hyaluronan on experimental osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1998;6(1):1–9.
Smith MM, Cake MA, Ghosh P, Schiavinato A, Read RA, Little CB. Significant synovial pathology in a meniscectomy model of osteoarthritis: modification by intra-articular hyaluronan therapy. Rheumatology (Oxford). 2008;47(8):1172–8.
Smith MM, Russell AK, Schiavinato A, Little CB. A hexadecylamide derivative of hyaluronan (HYMOVIS(R)) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan. J Inflamm (Lond). 2013;10:26.
Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis Cartilage. 1999;7(2):182–90.
Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Hyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis model. J Orthop Res. 2001;19(3):500–3.
Tanaka M, Masuko-Hongo K, Kato T, Nishioka K, Nakamura H. Suppressive effects of hyaluronan on MMP-1 and RANTES production from chondrocytes. Rheumatol Int. 2006;26(3):185–90.
Waddell DD, Kolomytkin OV, Dunn S, Marino AA. Hyaluronan suppresses IL-1beta-induced metalloproteinase activity from synovial tissue. Clin Orthop Relat Res. 2007;465:241–8.
Yasuda T. Nuclear factor-kappaB activation by type II collagen peptide in articular chondrocytes: its inhibition by hyaluronan via the receptors. Mod Rheumatol. 2013;23(6):1116–23.
Yasui T, Akatsuka M, Tobetto K, Hayaishi M, Ando T. The effect of hyaluronan on interleukin-1 alpha-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents Actions. 1992;37(1-2):155–6.
Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68(6):1051–8.
Yoshimi T, Kikuchi T, Obara T, Yamaguchi T, Sakakibara Y, Itoh H, et al. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop Relat Res. 1994;298:296–304.
Yoshioka M, Shimizu C, Harwood FL, Coutts RD, Amiel D. The effects of hyaluronan during the development of osteoarthritis. Osteoarthritis Cartilage. 1997;5(4):251–60.
Yu CJ, Ko CJ, Hsieh CH, Chien CT, Huang LH, Lee CW, et al. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid-regulated proteins involved in chondroprotective effect under oxidative stress. J Proteomics. 2014;99:40–53.
Zhang FJ, Gao SG, Cheng L, Tian J, Xu WS, Luo W, et al. The effect of hyaluronic acid on osteopontin and CD44 mRNA of fibroblast-like synoviocytes in patients with osteoarthritis of the knee. Rheumatol Int. 2013;33(1):79–83.
Zhou JL, Liu SQ, Qiu B, Hu QJ, Ming JH, Peng H. The protective effect of sodium hyaluronate on the cartilage of rabbit osteoarthritis by inhibiting peroxisome proliferator-activated receptor-gamma messenger RNA expression. Yonsei Med J. 2009;50(6):832–7.
Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26(12):1643–8.
Williams J. The effects of hyaluronic acid on fibronectin fragment mediated cartilage chondrolysis in skeletally mature rabbits. Osteoarthr Cartil. 2003;11(1):44–9.
Xu H, Ito T, Tawada A, Maeda H, Yamanokuchi H, Isahara K, et al. Effect of hyaluronan oligosaccharides on the expression of heat shock protein 72. J Biol Chem. 2002;277(19):17308–14.
Zhou J-L, Liu S-Q, Qiu B, Hu Q-J, Ming J-H, Peng H. Effects of hyaluronan on vascular endothelial growth factor and receptor-2 expression in a rabbit osteoarthritis model. J Orthop Sci. 2009;14(3):313–9.
Huang TL, Hsu HC, Yang KC, Yao CH, Lin FH. Effect of different molecular weight hyaluronans on osteoarthritis-related protein production in fibroblast-like synoviocytes from patients with tibia plateau fracture. J Trauma. 2010;68(1):146–52.
Amiel D, Toyoguchi T, Kobayashi K, Bowden K, Amiel ME, Healey RM. Long-term effect of sodium hyaluronate (Hyalgan) on osteoarthritis progression in a rabbit model. Osteoarthritis and cartilage / OARS, Osteoarthritis Res Soc. 2003;11(9):636–43.
Kim NH, Han CD, Lee HM, Yang IH. Effect of sodium hyaluronate on prevention of osteoarthritis. Yonsei Med J. 1991;32(2):139–46.
Liu J, Song W, Yuan T, Xu Z, Jia W, Zhang C. A comparison between platelet-rich plasma (PRP) and hyaluronate acid on the healing of cartilage defects. PLoS One. 2014;9(5), e97293.
Mihara M, Higo S, Uchiyama Y, Tanabe K, Saito K. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthritis Cartilage / OARS, Osteoarthritis Res Soc. 2007;15(5):543–9.
Qiu B, Liu SQ, Peng H, Wang HB. The effects of sodium hyaluronate on mRNA expressions of matrix metalloproteinase-1, -3 and tissue inhibitor of metalloproteinase-1 in cartilage and synovium of traumatic osteoarthritis model. Chin J Traumatol. 2005;8(1):8–12.
Ozkan FU, Ozkan K, Ramadan S, Guven Z. Chondroprotective effect of N-acetylglucosamine and hyaluronate in early stages of osteoarthritis--an experimental study in rabbits. Bull NYU Hosp Jt Dis. 2009;67(4):352–7.
Yang L, Zhang J, Wang G. The effect of sodium hyaluronate treating knee osteoarthritis on synovial fluid interleukin -1beta and clinical treatment mechanism. Pak J Pharm Sci. 2015;28(1 Suppl):407–10.
Yoshioka K, Yasuda Y, Kisukeda T, Nodera R, Tanaka Y, Miyamoto K. Pharmacological effects of novel cross-linked hyaluronate, Gel-200, in experimental animal models of osteoarthritis and human cell lines. Osteoarthritis Cartilage. 2014;22(6):879–87.
Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 2012;4(1):15–37.
Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein Cell. 2010;1(1):33–47.
Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998;61(2):125–35.
Bagga H, Burkhardt D, Sambrook P, March L. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33(5):946–50.
Bauer C, Baumgartner R, Hornof M, Halbwirth F, Niculescu-Morzsa E, Zwickl H, et al. Cross-linked hyaluronic acid scaffolds: a potential usage in cartilage regeneration? Osteoarthr Cartil. 2013;21:S312.
Forsey R, Fisher J, Thompson J, Stone M, Bell C, Ingham E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials. 2006;27(26):4581–90.
Frean S, Abraham L, Lees P. In vitro stimulation of equine articular cartilage proteoglycan synthesis by hyaluronan and carprofen. Res Vet Sci. 1999;67:181–8.
Han F, Ishiguro N, Ito T, Sakai T, Iwata H. Effects of sodium hyaluronate on experimental osteoarthritis in rabbit knee joints. Med Sci. 1999;62.
Homandberg GA, Ummadi V, Kang H. The role of insulin-like growth factor-I in hyaluronan mediated repair of cultured cartilage explants. Inflamm Res. 2004;53:8.
Hulmes D. Intra-articular hyaluronate in experimental rabbit osteoarthritis can prevent changes in cartilage proteoglycan content. Osteoarthr Cartil. 2004;12(3):232–8.
Kang Y, Eger W, Koepp H, Williams JM, Kuettner KE, Homandberg GA. Hyaluronan suppresses fibronectin fragment-mediated damage to human cartilage explant cultures by enhancing proteoglycan synthesis. J Orthop Res. 1999;17(6):858–69.
Kikuchi T, Yamada H, Fujikawa K. Effects of high molecular weight hyaluronan on the distribution and movement of proteoglycan around chondrocytes cultured in alginate beads. Osteoarthr Cartil. 2001;9(4):351–6.
Kobayashi K, Matsuzaka S, Yoshida Y, Miyauchi S, Wada Y, Moriya H. The effects of intraarticularly injected sodium hyaluronate on levels of intact aggrecan and nitric oxide in the joint fluid of patients with knee osteoarthritis. Osteoarthr Cartil. 2004;12(7):536–42.
Nishida Y, Knudson CB, Knudson W. Extracellular matrix recovery by human articular chondrocytes after treatment with hyaluronan hexasaccharides or Streptomyces hyaluronidase. Mod Rheumatol. 2003;13(1):62–8.
Smith Jr GN, Mickler EA, Myers SL, Brandt KD. Effect of intraarticular hyaluronan injection on synovial fluid hyaluronan in the early stage of canine post-traumatic osteoarthritis. J Rheumatol. 2001;28(6):1341–6.
Stove J, Gerlach C, Huch K, Gunther KP, Puhl W, Scharf HP. Effects of hyaluronan on proteoglycan content of osteoarthritic chondrocytes in vitro. J Orthop Res. 2002;20(3):551–5.
Wang C, Lin Y, Chiang B, Hou S. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr Cartil. 2006;14(12):1237–47.
Abatangelo G, Botti P, Del Bue M, Gei G, Samson JC, Cortivo R, et al. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. I. Biochemical results. Clin Orthop Relat Res. 1989;241:278–85.
Asari A, Mizuno S, Tanaka I, Sunose A, Kuriyama S, Miyazaki K, et al. Suppression of hyaluronan and prostaglandin E2 production in traumatic arthritic synovial cells by sodium hyaluronate. Connective Tissue. 1997;29:1–5.
Lisignoli G, Grassi F, Piacentini A, Cocchini B, Remiddi G, Bevilacqua C, et al. Hyaluronan does not affect cytokine and chemokine expression in osteoarthritic chondrocytes and synoviocytes. Osteoarthritis Cartilage. 2001;9(2):161–8.
Oliviero F, Scanu A, Ramonda R, Frallonardo P, Sfriso P, Dayer J, et al. Mechanisms involved in inhibition of inflammation in THP-1 cells by the hexadecylamide derivative of hyaluronic acid. Osteoarthr Cartil. 2014;22:S292–3.
Sezgin M, Demirel AÇ, Karaca C, Ortancıl Ö, Ülkar GB, Kanık A, et al. Does hyaluronan affect inflammatory cytokines in knee osteoarthritis? Rheumatol Int. 2004;25(4):264–9.
Sheehan KM, Delott LB, Day SM, Deheer DH. Hyalgan® has a dose-dependent differential effect on macrophage proliferation and cell death. J Orthop Res. 2003;21(4):744–51.
Yasuda T. Hyaluronan inhibits Akt, leading to nuclear factor-kappaB down-regulation in lipopolysaccharide-stimulated U937 macrophages. J Pharmacol Sci. 2011;115(4):509–15.
Campo GM, Avenoso A, D'Ascola A, Prestipino V, Scuruchi M, Nastasi G, et al. Inhibition of hyaluronan synthesis reduced inflammatory response in mouse synovial fibroblasts subjected to collagen-induced arthritis. Arch Biochem Biophys. 2012;518(1):42–52.
Campo GM, Avenoso A, Nastasi G, Micali A, Prestipino V, Vaccaro M, et al. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim Biophys Acta. 2011;1812(9):1170–81.
Schumacher HR, Paul C, Hitchon CA, El-Gabalawy H, Zonay L, Clayburne G, et al. Hyaluronate effects on synovium and synovial fluid. A prospective blinded study in patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(5):501–3.
Bell CJ, Ingham E, Fisher J. Influence of hyaluronic acid on the time-dependent friction response of articular cartilage under different conditions. Proc Inst Mech Eng H. 2006;220(1):23–31.
Ghosh P, Read R, Numata Y, Smith S, Armstrong S, Wilson D. The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. II. Cartilage composition and proteoglycan metabolism. Semin Arthritis Rheum. 1993;22(6 Suppl 1):31–42.
Obara T, Mabuchi K, Iso T, Yamaguchi T. Increased friction of animal joints by experimental degeneration and recovery by addition of hyaluronic acid. Clin Biomech (Bristol, Avon). 1997;12(4):246–52.
Waller KA, Zhang LX, Fleming BC, Jay GD. Preventing friction-induced chondrocyte apoptosis: comparison of human synovial fluid and Hylan G-F 20. J Rheumatol. 2012;39(7):1473–80.
Yu L-P, Yang H, Voschin E, Skrabut E. Viscoelastic properties and molecular weight of hylan G-F 20 compared with other commercial hyaluronan based viscosupplements. Osteoarthr Cartil. 2011;19(S1):S235.
Mori S, Naito M, Moriyama S. Highly viscous sodium hyaluronate and joint lubrication. Int Orthop. 2002;26(2):116–21.
Tang SF, Chen CP, Chen MJ, Pei YC, Lau YC, Leong CP. Changes in sagittal ground reaction forces after intra-articular hyaluronate injections for knee osteoarthritis. Arch Phys Med Rehabil. 2004;85(6):951–5.
Armstrong S, Read R, Ghosh P. The effects of intraarticular hyaluronan on cartilage and subchondral bone changes in an ovine model of early osteoarthritis. J Rheumatol. 1994;21(4):680–8.
Hiraoka N, Takahashi Y, Arai K, Honjo S, Nakawaga S, Tsuchida S, et al. Hyaluronan and intermittent hydrostatic pressure synergistically suppressed MMP-13 and Il-6 expressions in osteoblasts from OA subchondral bone. Osteoarthr Cartil. 2009;17(1):S97.
Hiraoka N, Takahashi KA, Arai Y, Sakao K, Mazda O, Kishida T, et al. Intra-articular injection of hyaluronan restores the aberrant expression of matrix metalloproteinase-13 in osteoarthritic subchondral bone. J Orthop Res. 2011;29(3):354–60.
Prasadam I, Crawford R, Xiao Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes -- possible pathogenic role in osteoarthritis. J Rheumatol. 2012;39(3):621–34.
Boettger MK, Kummel D, Harrison A, Schaible HG. Evaluation of long-term antinociceptive properties of stabilized hyaluronic acid preparation (NASHA) in an animal model of repetitive joint pain. Arthritis Res Ther. 2011;13(4):R110.
Gomis A, Miralles A, Schmidt RF, Belmonte C. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthr Cartil. 2009;17(6):798–804.
Gotoh S, Onaya J, Abe M, Miyazaki K, Hamai A, Horie K, et al. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann Rheum Dis. 1993;52(11):817–22.
Pena Ede L, Sala S, Rovira JC, Schmidt RF, Belmonte C. Elastoviscous substances with analgesic effects on joint pain reduce stretch-activated ion channel activity in vitro. Pain. 2002;99(3):501–8.
Dougados M. Sodium hyaluronate therapy in osteoarthritis: arguments for a potential beneficial structural effect. Semin Arthritis Rheum. 2000;30(2 Suppl 1):19–25.
Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9.
Karbownik MS, Nowak JZ. Hyaluronan: towards novel anti-cancer therapeutics. Pharmacol Rep. 2013;65(5):1056–74.
Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013;9(7):7081–92.
Harris E, Weigel PH. Functional Aspects of the Hyaluronan and Chondroitin Sulfate Receptors. In: Raton B, ed: CRC Press; 2009:171-192.
Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32(1):10–37.
Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221–64.
Camenisch TD, McDonald JA. Hyaluronan: is bigger better? Am J Respir Cell Mol Biol. 2000;23(4):431–3.
Mizrahy S, Raz SR, Hasgaard M, Liu H, Soffer-Tsur N, Cohen K, et al. Hyaluronan-coated nanoparticles: the influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J Control Release. 2011;156(2):231–8.
Jevsevar DS, Jones DL, Matzkin EG, Manner PA, Mooar P, Schousboe JT, et al. American academy of orthopaedic surgeons. Treatment of osteoarthritis of the knee: evidence based guideline 2nd Edition. JBJS. 2013;95(20):1885–6. Full document at: http://www.aaos.org/Research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf.
Lee PB, Kim YC, Lim YJ, Lee CJ, Sim WS, Ha CW, et al. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label, randomized, multicentre clinical trial. J Int Med Res. 2006;34(1):77–87.
Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2006;14(2):154–62.
Chen AL, Desai P, Adler EM, Di Cesare PE. Granulomatous inflammation after Hylan G-F 20 viscosupplementation of the knee : a report of six cases. J Bone Joint Surg Am. 2002;84-A(7):1142–7.
Zardawi IM, Chan I. Synvisc perisynovitis. Pathology. 2001;33(4):519–20.
Acknowledgements
This review is funded by Ferring Pharmaceuticals Inc. The authors would like to thank Mark Phillips and Global Research Solutions Inc. for their assistance in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Roy D. Altman: Consultant: Cytori, DuPuy (Bioventus Global), Ferring, Flexion, Iroko, McNeil, Novartis, Oletec, Pfizer, QMed, Rotta, Strategic Science and Technologies, Teva.
Speaker: Ferring, Iroko.
Ajay Manjoo: No conflicts of interest
Anke Fierlinger: Paid employee of Ferring Pharmaceuticals Inc.
Faizan Niazi: Paid employee of Ferring Pharmaceuticals Inc.
Mathew Nicholls: Serves on advisory boards for Ferring.
Authors’ contributions
AF and FN participated in the design and development of the study. RDA provided interpretation of the data, and significant writing contributions to the manuscript. All authors provided critical review and revisions to the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Altman, R., Manjoo, A., Fierlinger, A. et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord 16, 321 (2015). https://doi.org/10.1186/s12891-015-0775-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-015-0775-z