Abstract
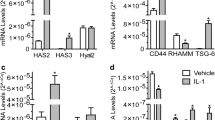
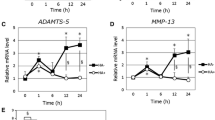
The purpose of the study was to examine the effects of hyaluronan (HA) on human chondrocytes in terms of production of MMP-1 and RANTES. Chondrocytes were obtained from patients with osteoarthritis (OA) or femoral neck fracture (control). Chondrocytes in monolayer culture were treated with various molecular weights (1.2, 50, 800 and 1,900 kD) of HA and then stimulated with IL-1β. Production and expression of MMP-1 and RANTES were quantified by ELISA and real-time polymerase chain reaction (PCR). The response was blocked by anti-CD44 antibody. Production of MMP-1 was significantly suppressed by both 800- and 1,900-kD HA, while production of RANTES was suppressed by 1,900-kD HA. Expression of MMP-1 and RANTES mRNA was inhibited by 1,900-kD HA. Suppressive effects of HA on production of MMP-1 were canceled by treatment of anti-CD44 antibody. Higher CD44 expression was found in OA chondrocytes than in those of control. High-molecular-weight HA suppressed MMP-1 and RANTES production, mediated partly by CD44–HA interaction.




Similar content being viewed by others
References
Laurent TC, Fraser JR (1992) Hyaluronan. FASEB J 6:2397–2404
Termeer C, Sleeman JP, Simon JC (2003) Hyaluronan—magic glue for the regulation of the immune response? Trends Immunol 24:112–114
McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW (1996) Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest 98:2403–2413
Kobayashi H, Suzuki M, Kanayama N, Nishida T, Takigawa M, Terao T (2002) CD44 stimulation by fragmented hyaluronic acid induces upregulation of urokinase-type plasminogen activator and its receptor and subsequently facilitates invasion of human chondrosarcoma cells. Int J Cancer 102:379–389
Shimizu M, Yasuda T, Nakagawa T, Yamashita E, Julovi SM, Hiramitsu T, Nakamura T (2003) Hyaluronan inhibits matrix metalloproteinase-1 production by rheumatoid synovial fibroblasts stimulated by proinflammatory cytokines. J Rheumatol 30:1164–1172
Hamburger MI, Lakhanpal S, Mooar PA, Oster D (2003) Intra-articular hyaluronans: a review of product-specific safety profiles. Semin Arthritis Rheum 32:296–309
Miltner O, Schneider U, Siebert CH, Niedhart C, Niethard FU (2002) Efficacy of intraarticular hyaluronic acid in patients with osteoarthritis—a prospective clinical trial. Osteoarthritis Cartilage 10:680–686
Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA (1997) Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum 40:2065–2074
Nakamura H, Shibakawa A, Tanaka M, Kato T, Nishioka K (2004) Effects of glucosamine hydrochloride on the production of prostaglandin E2, nitric oxide and metalloproteases by chondrocytes and synoviocytes in osteoarthritis. Clin Exp Rheumatol 22:293-299
Van de Loo FA, Joosten LA, van Lent PL, Arntz OJ, van den Berg WB (1995) Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum 38:164–172
Yuan GH, Masuko-Hongo K, Sakata M, Tsuruha J, Onuma H, Nakamura H, Aoki H, Kato T, Nishioka K (2001) The role of C–C chemokines and their receptors in osteoarthritis. Arthritis Rheum 44:1056–1070
Yasui T, Akatsuka M, Tobetto K, Hayaishi M, Ando T (1992) The effect of hyaluronan on interleukin-1 alpha-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents Actions 37:155–156
Akatsuka M, Yamamoto Y, Tobetto K, Yasui T, Ando T (1993) In vitro effects of hyaluronan on prostaglandin E2 induction by interleukin-1 in rabbit articular chondrocytes. Agents Actions 38:122–125
Fukuda K, Dan H, Takayama M, Kumano F, Saitoh M, Tanaka S (1996) Hyaluronic acid increased proteoglycan synthesis in bovine articular cartilage in the presence of interleukin-1. J Pharm Exp Ther 277:1672–1675
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K et al (1986) Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 29:1039–1049
Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M (1997) Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 24:365–71
Geuerassimov A, Zhang Y, Cartman A, Rosenberg LC, Esdaile J, Fitzcharles MA, Poole R (1999) Immune responses to cartilage link protein and the G1 domain of proteoglycan aggrecan in patients with osteoarthritis. Arthritis Rheum 42:527–533
Nakamura H, Yoshino S, Tsuruha J, Nishioka K (1999) T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthritis Cart 7:401–402
Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y (2000) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Am Rheum Dis 59:455–461
Lo GH, LaValley M, McAlindon T, Felson DT (2003) Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA 17(290):3115–3121
Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T (2004) Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum 50:516–525
Liu D, Sy MS (1997) Phorbol myristate acetate stimulates the dimerization of CD44 involving a cysteine in the transmembrane domain. J Immunol 15(159):2702–2711
Culty M, Nguyen HA, Underhill CB (1992) The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol 116:1055–1062
Flannery CR, Little CB, Hughes CE, Caterson B (1998) Expression and activity of articular cartilage hyaluronidases. Biochem Biophys Res Commun 29(251):824–829
Turley EA, Noble PW, Bourguignon LY (2002) Signaling properties of hyaluronan receptors. J Biol Chem 15(277):4589–4592
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, M., Masuko-Hongo, K., Kato, T. et al. Suppressive effects of hyaluronan on MMP-1 and RANTES production from chondrocytes. Rheumatol Int 26, 185–190 (2006). https://doi.org/10.1007/s00296-004-0547-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-004-0547-9