Abstract
Perivascular adult stem cells have been isolated from several tissues, including the adult human brain. They have unique signatures resembling both pericytes and mesenchymal stem cells. Understanding the nature of these cells in their specific vascular niches is important to determine their clinical potential as a new adult stem cell source. Indeed, they have promising features in vitro in terms of multipotency, immunomodulation and secretion of growth factors and cytokines. However, their in vivo function is less known as yet. Recent emerging data show a crucial role of perivascular mesenchymal stem cells in tissue homeostasis and repair. Furthermore, these cells may play an important role in adult stem cell niche regulation and in neurodegeneration. Here we review the recent literature on perivascular mesenchymal stem cells, discuss their different in vitro functions and highlight especially the specific properties of brain-derived perivascular mesenchymal stem cells. We summarize current evidence that suggests an important in vivo function of these cells in terms of their regenerative potential that may indicate a new target cell for endogenous tissue regeneration and repair.
Similar content being viewed by others
Review
Adult stem cells
Adult stem cells (ASCs) are found in almost all organs of the postnatal human body. They reside in the perivascular niche, a specific microenvironment that allows ASCs to retain their multi-lineage potential and self-renewal capacity[1, 2]. The perivascular niche consists of ASCs, neighbouring cells and extracellular matrix[1, 3, 4].
Adult stem cells are a source for organ-specific cell replacement either during the normal cell turnover or under pathological conditions[5, 6]. These stem cells often remain dormant until they are activated by the body’s need to maintain tissues, or in response to disease or tissue injury. Some ASC types, such as hematopoietic stem cells (HSCs) or enteric stem cells, have a high proliferation rate, whereas in other organs, ASCs only divide under certain conditions, stimulated by injury for example.
In contrast to embryonic stem cells, the differentiation potential of ASCs is regarded as more restricted, usually to the cells of the tissue in which they reside. This suggests that the differentiation of an ASC into a specialized cell might be dependent on the surrounding tissue. However, this classical paradigm of tissue-specific differentiation capacity has been challenged by observations of a different degree of plasticity in some adult tissues that has resulted in differentiation beyond tissue boundaries[5].
Mesenchymal stem cells
One ASC type that has specifically attracted attention during the past years are mesenchymal stem cells (MSCs)[7–12]. Friedenstein and co-workers[13] were the first to describe MSCs, originally termed mesenchymal stromal cells, as a rare population of plastic-adherent cells that could be isolated from the bone marrow but was different from HSCs[13]. Mesenchymal stem cells are isolated by adherence to plastic, possess a high proliferative potential and are characterized by the expression of a panel of surface markers[14] and their capacity to differentiate along mesodermal lineages such as osteoblasts, chondrocytes and adipocytes[15]. They have gained interest because they are not only multipotent, they also support hematopoiesis[16–18], are immunomodulatory[19–23] and have an intriguing pro-regenerative capacity due to the secretion of different growth factors and mitogens[12, 23].
Mesenchymal stem cells reside in the perivascular niche
Interestingly, sources for MSCs are not restricted to the bone marrow. Indeed, MSCs have been isolated from several tissues in different species[7, 10] but also from different human tissues and organs[24–26] including bone marrow[27], dental pulp[27, 28], adipose tissue[29], umbilical cord Wharton’s jelly[30], placenta[31] and recently also from the adult human brain[32]. Importantly, these MSCs are located in the perivascular niche and exhibit similarities to pericytes in terms of phenotype, gene expression and differentiation capacity[25, 26, 32].
Evidence, that MSCs and pericytes are biologically related had remained indirect for a long time, but a more systematic analysis of their association has only recently been made[25, 26, 33, 34]. Now it has been shown that MSCs may reside in the perivascular compartment and have characteristics identical to a subclass of pericytes[10, 24–26, 32, 34]. However, pericytes around capillaries are suggested not to be the only ancestors of MSC’s[33]. Adventitial cells that reside around larger vessels also natively express MSC surface markers[35, 36].
Pericytes
Pericytes are a heterogeneous cell population in the vascular niche[37], that line the abluminal side of endothelial cells in the perivascular space and are embedded within a shared basement membrane[38, 39]. They span the entire microvasculature. The phenotypic identification of pericytes is rather difficult due to the lack of one specific pericyte marker. Therefore, besides their location, a panel of different markers is usually used to identify pericytes[38, 40–42].
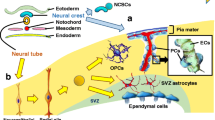
This diversity in pericyte marker expression may be due to differences in tissue location, vessel size or embryonic origin. It is generally proposed that pericytes are either mesodermal or neural crest-derived[43, 44], depending on their location in any given organ. In addition to their multiple embryonic origins, pericytes may develop from several adult cell types[38, 42, 45]. In the resting stage, pericytes are quiescent slow-cycling cells[46]. Once isolated from different tissues, pericytes have the capacity to proliferate and differentiate into different cell types in vitro.
Perivascular mesenchymal stem cells - a novel stem cell in the human brain
For many decades, the adult brain, in contrast to other tissues, was thought to not be capable of regeneration. However, it is now widely accepted that the adult human brain contains neural progenitors[47–53]. In the brain, adult neural stem cells are also found in specialized vascular niches, mainly in the neurogenic zones, the subventricular zone and the subgranular zone of the dentate gyrus[54–57]. In these vascular niches, the neural stem cells contact the vasculature at the sites that lack astrocyte endfeet and pericyte coverage[58].
Neural progenitor cells could also be derived from a variety of adult brain regions other than the known neurogenic zones[49, 52, 59, 60]. Human adult progenitor cells isolated from non-neurogenic regions multiply in vitro and give rise to cells with the characteristics of neurons, astrocytes, and oligodendrocytes[59, 61, 62].
Analyzing human brain tissue from biopsies and temporal lobectomies, we have identified a novel adult stem cell with mesenchymal characteristics located around small blood vessels in the human brain that is different from the previously described neural stem and/or progenitor cells[32]. These perivascular cells expressing mesenchymal (CD105, CD13) and pericyte markers (PDGFR-β) are mainly located at vascular branching points. Some of the pericytes co-expressing MSC markers are proliferating cells. Isolated cells were further purified by fluorescence-activated cell sorting (FACS), gating them positively for CD105, CD13, and negatively for the hematopoietic marker CD45 and the endothelial marker CD31. The expanded purified cells exhibited a marker signature for both MSCs and pericytes in vitro (CD73, CD90, CD13, CD106, CD49d, PDGFR-β, RGS5, α-SMA, NG2). Cells were negative for hematopoietic, endothelial, and glial markers. Most importantly, the isolated cells did not express any of the neural progenitor markers that are typical for adult neural stem cells (CD133, SOX1, NGN2, PAX6 and Musashi) (Table1).
Isolated perivascular MSCs from the adult human brain undergo self-renewal in vitro and give rise to single-cell-derived clones that are indistinguishable from polyclonal perivascular MSCs in terms of adherence, morphology, proliferation, and surface antigen expression. Surprisingly, the capacity of these brain-derived perivascular MSCs was far superior to our initial expectations (Figure1). Single-cell-derived clones gave rise to adipocytes, chondroblasts and osteoblast when exposed to the appropriate inductive signals, a feature that had been described for both MSC[15, 25] and pericytes[25, 63–66].
The adult human brain vascular niche contains a novel progenitor with multi-lineage capacity that appears to represent both MSCs and pericytes. These progenitor cells give rise to both neuronal lineage (astrocyte, oligodendrocytes, and neurons) and mesodermal lineage (adipocytes, chondroblasts, and osteoblasts) at the clonal level.
Most interestingly, when isolated perivascular MSCs were exposed to glial induction medium, the cells differentiated into oligodendrocytes or astrocytes, pericyte-specific antigens were downregulated and cells expressed glial fibrillary acidic protein (GFAP).
Furthermore, upon neuronal induction, the same perivascular MSC clones downregulated mRNA for pericyte markers (α-SMA, Nestin, RGS5, NG2 and PDGFR-β) and upregulated mRNA for neuronal transcription factors (NeuroD1, Pax6, Tbr1, Tbr2) and neuronal markers (DCX, Tuj1) and consistently expressed neuron-specific proteins (DCX; HuC/D, Map2, Tuj1, NSE). A proportion of neurons expressed the synaptic marker PSD95 and GABA A-receptor, indicating a more mature neuronal phenotype. Cells exhibited typical electrophysiological features of immature neurons, consistent with the slow maturation of human neurons.
Thus, perivascular MSCs have a broader stem cell potential than classical neural stem cells. Moreover, perivascular MSCs are not restricted to a certain perivascular niche in neurogenic regions but could be easily isolated from non-neurogenic regions in the brain. Thus, the perivascular MSC is a novel, unique population distinct from the neural stem cells in the adult brain that has both neuroectodermal and mesodermal differentiation capacity in vitro. This differentiation capacity was retained in long-term proliferating cultures.
The most intriguing question to be answered now is obviously which role these cells play for disease and repair in vivo and whether this reflects their in vitro potential.
Regenerative potential of perivascular mesenchymal stem cells
Perivascular MSCs possess both MSC and pericyte features. Both cell types have been described to have different properties that may play a role in regeneration.
Mesenchymal stem cells in vitro have shown several interesting features such as multipotentiality, immunomodulation, and pro-regenerative capacities[9, 12, 15]. Due to these properties, MSCs have become one of the most promising ASC types and are currently being tested in several clinical trials. Indeed, MSCs are explored as a treatment for Crohn’s disease[67–70], for acute graft versus host reaction[71–73], myocardial infarct[74–76], limb ischemia[77, 78], osteogenesis imperfecta[79–81], and for neurological disorders such as stroke[82–84], cerebral palsy[85], amyotrophic lateral sclerosis[86, 87] and multiple sclerosis[88–91]. A current search gives a total of 298 clinical studies using different sources of MSCs and mesenchymal stromal cells (http://www.clinicaltrials.gov). In most of these clinical trials, MSCs are used in an autologous and allogenic ex vivo transplantation setting for repair.
Akin to MSCs, pericytes have been reported to be able to differentiate into osteoblasts[25, 63, 64], chondrocytes, adipocytes[25, 65, 66], muscle cells[25, 92], but also neuroectodermal lineages[32, 93].
It remains to be answered whether and to what extent these described in vitro properties reflect the in vivo function of perivascular MSCs as these properties might be altered upon isolation and culture in vitro.
Multipotentiality in vivo
In vivo studies are rare due to the ambiguity in markers, but there is some promising evidence that suggest that pericytes may serve as an in vivo source of stem or progenitor cells for adult tissue repair[94, 95]. Under pathological conditions, a tissue-specific differentiation capacity of pericytes has been observed. Pericytes differentiate into adipocytes during fat tissue injury[29, 96], into chondroblasts and bone after bone injury[64] and are the progenitors of Leydig cells of the testis[97]. Genetic lineage tracing reveals that pericytes form odontoblasts during tooth growth and damage in vivo[46]. They also contribute to myocytes in skeletal muscle during development and repair[98] and are more frequent in muscles of myopathic patients compared to controls[99]. Furthermore, pericytes are progenitors of follicular dendritic cells in lymphoid follicles[100], they are the origin of myofibroblasts in kidney fibrosis[101], and at least a subtype of pericytes contributes to scar formation in the spinal cord[102]. Resident perivascular MSC give rise to myofibroblasts following lens injury and contribute to fibrogenesis in human lung allografts[103, 104] (for summary see Table2).
Immunomodulation
Besides their ability to differentiate into cell types from different lineages, isolated MSCs also have an immunomodulatory role[12, 21–23].
They have been shown to have an inhibitory effect on lymphocytes[105, 106], on B-cells[107], dendritic cells[108] and natural killer cells[109, 110]. Furthermore, MSCs modulate the inflammatory response of microglial cells, resident immunocompetent cells in the brain[111]. Mesenchymal stem cells hereby inhibit the expression and release of inflammatory molecules and stress-associated proteins and change microglial cells from a detrimental to a more neuroprotective phenotype[112]. Thus, these immunomodulatory features of MSCs may have an indirect neuroprotective effect[113]. Mesenchymal stem cells lead to amelioration in multiple sclerosis through inhibition of the pathogenic immune response and the release of neuroprotective molecules[22]. They have also been shown to suppress ischemia-induced inflammation[114]. The neuroprotective effect of MSCs in stroke was also mediated via a change in resident microglia to a more neuroprotective type[115]. Furthermore, in a model of Parkinson’s disease, dopaminergic cell death that was induced by activated microglia could be prevented by grafting MSCs[116]. Similar results, demonstrating decreased activation of astrocytes and microglia by MSCs in a mouse model of multiple system atrophy have recently been reported[117, 118].
Similarly to MSCs, pericytes have been described to regulate T-cell activation, recruit T- and B-lymphocytes to areas of tissue injury[119, 120] and control transmigration of thymocytes from the thymus across the blood vessel wall[121]. In addition, brain pericytes have been shown to secrete different cytokines in vitro[122].
Should these immunomodulatory features be present on resident perivascular MSCs in vivo, they could indeed play a primary role in inhibiting immunosurveillance and thereby establish a regenerative environment[11].
Pro-regeneration
A third, and most important feature of isolated MSCs is their pro-regenerative capacity. Mesenchymal stem cells secrete a large number of cytokines, growth factors, mitogens and angiogenic factors[12, 95]. This raises the question of whether MSCs could also be promoting a regenerative environment by production of growth factors and cytokines in vivo[11].
The most interesting scientific question now is whether their in vivo perivascular counterparts hold similar properties mentioned above. It is conceivable that resident perivascular MSCs support the local ASC niche either directly by differentiating into tissue-specific cells as indicated above, or indirectly, by regulating the stem cell niche[123]. Interestingly, pericytes have been shown to contribute to tissue repair and wound healing in vivo by substantially enhancing the tissue-regenerative capacity of human epidermal cells[124].
The HSC niche, where MSCs were first identified, is currently the best characterized example of an ASC niche in vivo function of resident MSC in the HSC niche was recently revealed by lineage-tracing using nestin as a marker for MSC. This data suggests that resident MSCs are responsible for the maintenance of the HSC niche by regulating the proliferation and survival of HSCs[16].
Do perivascular mesenchymal stem cells/pericytes play a role in brain repair?
Whether the properties and functions of perivascular MSCs vary between tissues or whether these cells are biologically equivalent will need to be systematically evaluated. The diversity of pericytes is largely unexplored, but there are indications that pericytes in the brain may have specific potential and functions[119, 123, 125–127]. The brain is one of the most vascularized organs and pericytes have a higher density in the brain, and the brain has a lower endothelial/pericyte ratio compared to other organs[38]. Consistent with their higher density, pericytes appear to act as a key modulator of the neurovascular unit in the brain[123]. Neurovascular pericytes regulate the blood brain barrier[123, 126], capillary flow, angiogenesis[128] and immune responses[37, 39, 41, 129, 130]. Minor disturbances in the blood vessels can compromise neuronal performance because of the importance of the vasculature for neuronal homeostasis, delivery of oxygen and nutrients, removal of metabolic waste and preservation of the neuronal microenvironment[131]. This is reflected in the fact that vascular damage in pericyte-deficient mice preceeds neuronal damage and neurodegeneration, suggesting that neurodegeneration may develop secondary to disturbances in cerebral vascular homeostasis[127]. Thus, microvascular dysfunction due to pericyte degeneration may initiate neurodegenerative changes[123]. Resident perivascular MSCs may thus regulate the local ASC niche. Another hypothesis could be that pericytes respond to injury by tissue-specific differentiation as evident from other organs (Table2). Pericytes have been shown to migrate in response to traumatic brain injury[132]. Recent studies that have isolated brain pericytes indicate that the differentiation potential of brain-derived pericytes in vitro extends beyond the mesodermal lineage to the neuroectodermal lineage[32, 93]. This may at least partially reflect their inherent differentiation potential and could, in analogy to emerging studies in other tissues, possibly indicate their in vivo capacity.
However, the role that is played by these cells in brain development and repair remains most speculative and yet, represents one of the most fascinating questions to be raised. It now remains to be shown whether perivascular MSCs/pericytes resident in the brain have similar or equal functional characteristics in vivo, supporting the stem cell niche and controlling stem cell proliferation and differentiation. This could place resident perivascular MSCs in a crucial position for contributing to brain disease and regeneration, as much pathology has been associated with a dysregulation of the stem cell niche.
We believe that the properties of these cells observed in other tissues may also apply to the brain. Thus, from a therapeutic perspective, resident MSCs emerge as an extremely promising target or agent for tissue regeneration.
Conclusion
In a time when the world’s population is aging, the health burden of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease but also conditions such as stroke is constantly increasing. To manage the larger number of patients and the connected health costs, brain research will have to direct a sharp focus towards developing neurorestorative and neuroprotective treatments.
In the next few years, the focus will be on studying the in vivo function of the newly discovered perivascular stem cells in the brain. Evidence from in vitro work and in vivo observations in other tissues gives hope that these perivascular stem cells may play a key role for regeneration of the brain in response to trauma, injury or degeneration. The aim is to control and enhance any pro-regenerative capacities of these cells by delivering therapies targeted at stimulating the cells to relocate to sites of injury or damage.
To understand and harness the reparative potential of ASCs in the brain will be key in setting the course for future research on neurodegeneration and neurorestoration.
Abbreviations
- ASCs:
-
Adult Stem Cells
- MSCs:
-
Mesenchymal Stem Cells
- HSCs:
-
Hematopoietic Stem Cells
- CD105:
-
Endoglin cell membrane glycoprotein
- CD13:
-
Cluster differentiation marker
- PDGFRβ:
-
Platelet-Derived Growth Factor Receptor beta
- FACS:
-
Fluorescence-Activated Cell Sorting
- CD45:
-
Leukocyte common antigen
- CD31:
-
Pan-endothelial marker
- CD73:
-
Ecto-5′-nucleotidase (NT5E). GPI-anchored purine enzyme expressed on the surface of human T and B lymphocytes
- CD90:
-
Thymocyte differentiation antigen 1 (Thy-1)
- CD106:
-
Vascular cell adhesion protein 1 (VCAM-1)
- CD49d:
-
Alpha subunit of integrin alpha4beta1
- RGS5:
-
Regulator of G-protein Signaling 5
- α-SMA:
-
Alpha Smooth Muscle Actin
- NG2:
-
Chondroitin Sulfate Proteoglycan
- NGN2:
-
Neurogenin 2
- CD133:
-
Prominin 1
- SOX1:
-
Sox gene 1
- PAX6:
-
Paired box protein 6
- GFAP:
-
Glial Fibrillary Acidic Protein
- Tbr1:
-
T-box brain 1 transcription factor
- Tbr2:
-
T-box brain 2 transcription factor
- DCX:
-
Doublecortin
- Tuj1:
-
Neuronal class III beta-tubulin
- HuC/D:
-
Anti human neuronal protein HuC/D
- Map2:
-
Microtubule-associated protein 2
- NSE:
-
Neuron-specific Enolase
- PSD95:
-
Postsynaptic Density Protein 95
- GABA:
-
Gamma-Aminobutyric Acid
- Kir6.1:
-
Potassium channel subunit
- A2B5:
-
Ganglioside marker
- S100b:
-
S100 calcium-binding protein
- GLAST:
-
Astrocyte-specific transporter
- O4:
-
Oligodendrocyte marker
- CD34:
-
Single chain transmembrane glycoprotein selectively expressed on human lymphoid and myeloid hematopoietic progenitors cells
- CD166:
-
Activated leukocyte cell adhesion molecule
- CD56:
-
Neural cell adhesion molecule (N-CAM)
- CD14:
-
Cluster of differentiation 14, cell surface receptor and differentiation marker
- CD11b:
-
Macrophage antigen 1 (Mac-1)
- Stro1:
-
Mesenchymal/stromal stem cell marker 1
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- Foxd1:
-
Forkhead box transcription factor 1.
References
Wurmser AE, Palmer TD, Gage FH: Neuroscience. Cellular interactions in the stem cell niche. Science 2004, 304: 1253–1255. 10.1126/science.1099344
Goldman SA, Chen Z: Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci 2011, 14: 1382–1389. 10.1038/nn.2963
Diaz-Flores L Jr, Madrid JF, Gutierrez R, Varela H, Valladares F, Alvarez-Arguelles H, Diaz-Flores L: Adult stem and transit-amplifying cell location. Histol Histopathol 2006, 21: 995–1027.
Nikolova G, Strilic B, Lammert E: The vascular niche and its basement membrane. Trends Cell Biol 2007, 17: 19–25. 10.1016/j.tcb.2006.11.005
Korbling M, Estrov Z: Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med 2003, 349: 570–582. 10.1056/NEJMra022361
Slack JM: Stem cells in epithelial tissues. Science 2000, 287: 1431–1433. 10.1126/science.287.5457.1431
Bianco P, Robey PG, Simmons PJ: Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2008, 2: 313–319. 10.1016/j.stem.2008.03.002
Nombela-Arrieta C, Ritz J, Silberstein LE: The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 2011, 12: 126–131. 10.1038/nrm3049
Bieback K, Wuchter P, Besser D, Franke W, Becker M, Ott M, Pacher M, Ma N, Stamm C, Kluter H, Muller A, Ho AD: Mesenchymal stromal cells (MSCs): science and f(r)iction. J Mol Med (Berl) 2012, 90: 773–782. 10.1007/s00109-012-0915-y
da Silva Meirelles L, Caplan AI, Nardi NB: In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008, 26: 2287–2299. 10.1634/stemcells.2007-1122
Caplan AI: Why are MSCs therapeutic? New data: new insight. J Pathol 2009, 217: 318–324. 10.1002/path.2469
Caplan AI, Correa D: The MSC: an injury drugstore. Cell Stem Cell 2011, 9: 11–15. 10.1016/j.stem.2011.06.008
Friedenstein AJ, Chailakhjan RK, Lalykina KS: The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970, 3: 393–403.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8: 315–317. 10.1080/14653240600855905
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284: 143–147. 10.1126/science.284.5411.143
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS: Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466: 829–834. 10.1038/nature09262
Wilson A, Trumpp A: Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 2006, 6: 93–106. 10.1038/nri1779
Kiel MJ, Morrison SJ: Maintaining hematopoietic stem cells in the vascular niche. Immunity 2006, 25: 862–864. 10.1016/j.immuni.2006.11.005
Le Blanc K, Pittenger M: Mesenchymal stem cells: progress toward promise. Cytotherapy 2005, 7: 36–45. 10.1080/14653240510018118
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM: Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99: 3838–3843. 10.1182/blood.V99.10.3838
Aggarwal S, Pittenger MF: Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105: 1815–1822. 10.1182/blood-2004-04-1559
Uccelli A, Moretta L, Pistoia V: Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008, 8: 726–736. 10.1038/nri2395
Singer NG, Caplan AI: Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011, 6: 457–478. 10.1146/annurev-pathol-011110-130230
da Silva Meirelles L, Chagastelles PC, Nardi NB: Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006, 119: 2204–2213. 10.1242/jcs.02932
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B: A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3: 301–313. 10.1016/j.stem.2008.07.003
Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA: Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 2008, 36: 642–654. 10.1016/j.exphem.2007.12.015
Shi S, Gronthos S: Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 2003, 18: 696–704. 10.1359/jbmr.2003.18.4.696
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S: Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364: 149–155. 10.1016/S0140-6736(04)16627-0
Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM: White fat progenitor cells reside in the adipose vasculature. Science 2008, 322: 583–586. 10.1126/science.1156232
Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE: Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 2005, 23: 220–229. 10.1634/stemcells.2004-0166
Robin C, Bollerot K, Mendes S, Haak E, Crisan M, Cerisoli F, Lauw I, Kaimakis P, Jorna R, Vermeulen M, Kayser M, van der Linden R, Imanirad P, Verstegen M, Nawaz-Yousaf H, Papazian N, Steegers E, Cupedo T, Dzierzak E: Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell 2009, 5: 385–395. 10.1016/j.stem.2009.08.020
Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, Anisimov SV, Renstrom E, Svensson M, Haegerstrand A, Brundin P: The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One 2012, 7: e35577. 10.1371/journal.pone.0035577
Corselli M, Chen CW, Crisan M, Lazzari L, Peault B: Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol 2010, 30: 1104–1109. 10.1161/ATVBAHA.109.191643
Caplan AI: All MSCs are pericytes? Cell Stem Cell 2008, 3: 229–230. 10.1016/j.stem.2008.08.008
Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B: The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 2012, 21: 1299–1308. 10.1089/scd.2011.0200
Chen CW, Corselli M, Peault B, Huard J: Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol 2012, 2012: 597439.
Hirschi KK, D’Amore PA: Pericytes in the microvasculature. Cardiovasc Res 1996, 32: 687–698.
Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L Jr: Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 2009, 24: 909–969.
Dore-Duffy P, Cleary K: Morphology and properties of pericytes. Methods Mol Biol 2011, 686: 49–68. 10.1007/978-1-60761-938-3_2
Armulik A, Abramsson A, Betsholtz C: Endothelial/pericyte interactions. Circ Res 2005, 97: 512–523. 10.1161/01.RES.0000182903.16652.d7
Krueger M, Bechmann I: CNS pericytes: concepts, misconceptions, and a way out. Glia 2010, 58: 1–10. 10.1002/glia.20898
Kamouchi M, Ago T, Kitazono T: Brain pericytes: emerging concepts and functional roles in brain homeostasis. Cell Mol Neurobiol 2011, 31: 175–193. 10.1007/s10571-010-9605-x
Etchevers HC, Vincent C, Le Douarin NM, Couly GF: The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001, 128: 1059–1068.
Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S: Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000, 408: 92–96. 10.1038/35040568
Armulik A, Genove G, Betsholtz C: Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011, 21: 193–215. 10.1016/j.devcel.2011.07.001
Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT: Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A 2011, 108: 6503–6508. 10.1073/pnas.1015449108
Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL: Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A 2000, 97: 14720–14725. 10.1073/pnas.97.26.14720
Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A: Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004, 427: 740–744. 10.1038/nature02301
Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA: Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 2003, 9: 439–447. 10.1038/nm837
Pagano SF, Impagnatiello F, Girelli M, Cova L, Grioni E, Onofri M, Cavallaro M, Etteri S, Vitello F, Giombini S, Solero CL, Parati EA: Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells 2000, 18: 295–300. 10.1634/stemcells.18-4-295
Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA: Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol 1999, 156: 333–344. 10.1006/exnr.1999.7028
Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J: Neural stem cells in the adult human brain. Exp Cell Res 1999, 253: 733–736. 10.1006/excr.1999.4678
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH: Neurogenesis in the adult human hippocampus. Nat Med 1998, 4: 1313–1317. 10.1038/3305
Alvarez-Buylla A, Lim DA: For the long run: maintaining germinal niches in the adult brain. Neuron 2004, 41: 683–686. 10.1016/S0896-6273(04)00111-4
Miller FD, Gauthier-Fisher A: Home at last: neural stem cell niches defined. Cell Stem Cell 2009, 4: 507–510. 10.1016/j.stem.2009.05.008
Gage FH: Mammalian neural stem cells. Science 2000, 287: 1433–1438. 10.1126/science.287.5457.1433
Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA: In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med 2000, 6: 271–277. 10.1038/73119
Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F: A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3: 279–288. 10.1016/j.stem.2008.07.025
Walton NM, Sutter BM, Chen HX, Chang LJ, Roper SN, Scheffler B, Steindler DA: Derivation and large-scale expansion of multipotent astroglial neural progenitors from adult human brain. Development 2006, 133: 3671–3681. 10.1242/dev.02541
Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH: Cell culture. Progenitor cells from human brain after death. Nature 2001, 411: 42–43. 10.1038/35075141
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J: Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999, 96: 25–34. 10.1016/S0092-8674(00)80956-3
Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G 2nd, Roy NS, Goldman SA: Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med 2004, 10: 93–97. 10.1038/nm974
Canfield AE, Sutton AB, Hoyland JA, Schor AM: Association of thrombospondin-1 with osteogenic differentiation of retinal pericytes in vitro. J Cell Sci 1996, 109(Pt 2):343–353.
Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE: Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res 1998, 13: 828–838. 10.1359/jbmr.1998.13.5.828
Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE: Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 2004, 110: 2226–2232. 10.1161/01.CIR.0000144457.55518.E5
Brachvogel B, Moch H, Pausch F, Schlotzer-Schrehardt U, Hofmann C, Hallmann R, von der Mark K, Winkler T, Poschl E: Perivascular cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development 2005, 132: 2657–2668. 10.1242/dev.01846
Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L: Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 2012, 61: 468–469. 10.1136/gutjnl-2011-300083
Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR: Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 2011, 60: 788–798. 10.1136/gut.2010.214841
Mannon PJ: Remestemcel-L: human mesenchymal stem cells as an emerging therapy for Crohn’s disease. Expert Opin Biol Ther 2011, 11: 1249–1256. 10.1517/14712598.2011.602967
Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW: Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 2010, 59: 1662–1669. 10.1136/gut.2010.215152
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O: Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008, 371: 1579–1586. 10.1016/S0140-6736(08)60690-X
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O: Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363: 1439–1441. 10.1016/S0140-6736(04)16104-7
Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE, Svinareva DA, Drize NJ, Savchenko VG: Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease-a phase II study. Stem Cells Int 2012, 2012: 968213.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S: Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001, 7: 430–436. 10.1038/86498
Chen CP, Lee YJ, Chiu ST, Shyu WC, Lee MY, Huang SP, Li H: The application of stem cells in the treatment of ischemic diseases. Histol Histopathol 2006, 21: 1209–1216.
Song H, Song BW, Cha MJ, Choi IG, Hwang KC: Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther 2010, 10: 309–319. 10.1517/14712590903455997
Liew A, O’Brien T: Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther 2012, 3: 28. 10.1186/scrt119
Lasala GP, Silva JA, Minguell JJ: Therapeutic angiogenesis in patients with severe limb ischemia by transplantation of a combination stem cell product. J Thorac Cardiovasc Surg 2012, 144: 377–382. 10.1016/j.jtcvs.2011.08.053
Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T: Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A 2002, 99: 8932–8937. 10.1073/pnas.132252399
Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK: Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999, 5: 309–313. 10.1038/6529
Otsuru S, Gordon PL, Shimono K, Jethva R, Marino R, Phillips CL, Hofmann TJ, Veronesi E, Dominici M, Iwamoto M, Horwitz EM: Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012, 120: 1933–1941. 10.1182/blood-2011-12-400085
Bang OY, Lee JS, Lee PH, Lee G: Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005, 57: 874–882. 10.1002/ana.20501
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD: Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011, 134: 1790–1807. 10.1093/brain/awr063
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY: A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28: 1099–1106. 10.1002/stem.430
Lee YH, Choi KV, Moon JH, Jun HJ, Kang HR, Oh SI, Kim HS, Um JS, Kim MJ, Choi YY, Lee YJ, Kim HJ, Lee JH, Son SM, Choi SJ, Oh W, Yang YS: Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med 2012, 10: 58. 10.1186/1479-5876-10-58
Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, Testa L, Stecco A, Tarletti R, Miglioretti M, Fava E, Nasuelli N, Cisari C, Massara M, Vercelli R, Oggioni GD, Carriero A, Cantello R, Monaco F, Fagioli F: Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol 2010, 223: 229–237. 10.1016/j.expneurol.2009.08.007
Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, Carriero A, Monaco F, Fagioli F: Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy 2012, 14: 56–60. 10.3109/14653249.2011.613929
Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S: Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 2012, 11: 150–156. 10.1016/S1474-4422(11)70305-2
Bonab MM, Sahraian MA, Aghsaie A, Karvigh SA, Hosseinian SM, Nikbin B, Lotfi J, Khorramnia S, Motamed MR, Togha M, Harirchian MH, Moghadam NB, Alikhani K, Yadegari S, Jafarian S, Gheini MR: Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther 2012. (Epud ahead of print) (Epud ahead of print)
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S: Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010, 67: 1187–1194. 10.1001/archneurol.2010.248
Connick P, Kolappan M, Patani R, Scott MA, Crawley C, He XL, Richardson K, Barber K, Webber DJ, Wheeler-Kingshott CA, Tozer DJ, Samson RS, Thomas DL, Du MQ, Luan SL, Michell AW, Altmann DR, Thompson AJ, Miller DH, Compston A, Chandran S: The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials 2011, 12: 62. 10.1186/1745-6215-12-62
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G: Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 2007, 9: 255–267. 10.1038/ncb1542
Dore-Duffy P, Katychev A, Wang X, Van Buren E: CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 2006, 26: 613–624. 10.1038/sj.jcbfm.9600272
Peault B: Are mural cells guardians of stemness?: From pluri- to multipotency via vascular pericytes. Circulation 2012, 125: 12–13. 10.1161/CIRCULATIONAHA.111.073445
Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, Peault B: Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev 2009, 20: 429–434. 10.1016/j.cytogfr.2009.10.014
Richardson RL, Hausman GJ, Campion DR: Response of pericytes to thermal lesion in the inguinal fat pad of 10-day-old rats. Acta Anat (Basel) 1982, 114: 41–57. 10.1159/000145577
Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D: Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol 2004, 167: 935–944. 10.1083/jcb.200409107
Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G: Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2011, 2: 499.
Diaz-Manera J, Gallardo E, de Luna N, Navas M, Soria L, Garibaldi M, Rojas-Garcia R, Tonlorenzi R, Cossu G, Illa I: The increase of pericyte population in human neuromuscular disorders supports their role in muscle regeneration in vivo. J Pathol 2012, 228: 544–553.
Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D, Schwarz P, Armulik A, Browning JL, Tallquist M, Buch T, Oliveira-Martins JB, Zhu C, Hermann M, Wagner U, Brink R, Heikenwalder M, Aguzzi A: Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 2012, 150: 194–206. 10.1016/j.cell.2012.05.032
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 2010, 176: 85–97. 10.2353/ajpath.2010.090517
Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J: A pericyte origin of spinal cord scar tissue. Science 2011, 333: 238–242. 10.1126/science.1203165
Badri L, Murray S, Liu LX, Walker NM, Flint A, Wadhwa A, Chan KM, Toews GB, Pinsky DJ, Martinez FJ, Lama VN: Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2011, 183: 1062–1070. 10.1164/rccm.201005-0742OC
Walker JL, Zhai N, Zhang L, Bleaken BM, Wolff I, Gerhart J, George-Weinstein M, Menko AS: Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proc Natl Acad Sci U S A 2010, 107: 13730–13735. 10.1073/pnas.0910382107
Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F: Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105: 2821–2827. 10.1182/blood-2004-09-3696
Le Blanc K, Ringden O: Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 2007, 262: 509–525. 10.1111/j.1365-2796.2007.01844.x
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A: Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107: 367–372. 10.1182/blood-2005-07-2657
Ramasamy R, Fazekasova H, EW L, Soeiro I, Lombardi G, Dazzi F: Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007, 83: 6.
Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L: Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107: 1484–1490. 10.1182/blood-2005-07-2775
Uccelli A, Pistoia V, Moretta L: Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 2007, 28: 219–226. 10.1016/j.it.2007.03.001
Zhou C, Zhang C, Chi S, Xu Y, Teng J, Wang H, Song Y, Zhao R: Effects of human marrow stromal cells on activation of microglial cells and production of inflammatory factors induced by lipopolysaccharide. Brain Res 2009, 1269: 23–30.
Giunti D, Parodi B, Usai C, Vergani L, Casazza S, Bruzzone S, Mancardi G, Uccelli A: Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells 2012, 30: 2044–2053. 10.1002/stem.1174
Saijo K, Glass CK: Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 2011, 11: 775–787. 10.1038/nri3086
Sheikh AY, Huber BC, Narsinh KH, Spin JM, van der Bogt K, de Almeida PE, Ransohoff KJ, Kraft DL, Fajardo G, Ardigo D, Ransohoff J, Bernstein D, Fischbein MP, Robbins RC, Wu JC: In vivo functional and transcriptional profiling of bone marrow stem cells after transplantation into ischemic myocardium. Arterioscler Thromb Vasc Biol 2012, 32: 92–102. 10.1161/ATVBAHA.111.238618
Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ: Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A 2008, 105: 14638–14643. 10.1073/pnas.0803670105
Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, Kim HO, Lee PH: Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 2009, 57: 13–23. 10.1002/glia.20731
Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK: Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS One 2011, 6: e19808. 10.1371/journal.pone.0019808
Park HJ, Bang G, Lee BR, Kim HO, Lee PH: Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy parkinsonism. Cell Transplant 2011, 20: 827–835. 10.3727/096368910X540630
Balabanov R, Beaumont T, Dore-Duffy P: Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J Neurosci Res 1999, 55: 578–587. 10.1002/(SICI)1097-4547(19990301)55:5<578::AID-JNR5>3.0.CO;2-E
Dulmovits BM, Herman IM: Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol 2012, 44: 1800–1812. 10.1016/j.biocel.2012.06.031
Zachariah MA, Cyster JG: Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 2010, 328: 1129–1135. 10.1126/science.1188222
Kovac A, Erickson MA, Banks WA: Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation 2011, 8: 139. 10.1186/1742-2094-8-139
Winkler EA, Bell RD, Zlokovic BV: Central nervous system pericytes in health and disease. Nat Neurosci 2011, 14: 1398–1405. 10.1038/nn.2946
Paquet-Fifield S, Schluter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, Brouard N, Zannettino A, Saunders N, Thompson N, Li J, Kaur P: A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest 2009, 119: 2795–2806.
Dore-Duffy P: Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 2008, 14: 1581–1593. 10.2174/138161208784705469
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C: Pericytes regulate the blood–brain barrier. Nature 2010, 468: 557–561. 10.1038/nature09522
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV: Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68: 409–427. 10.1016/j.neuron.2010.09.043
Gerhardt H, Betsholtz C: Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 2003, 314: 15–23. 10.1007/s00441-003-0745-x
Thomas WE: Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev 1999, 31: 42–57.
Balabanov R, Dore-Duffy P: Role of the CNS microvascular pericyte in the blood–brain barrier. J Neurosci Res 1998, 53: 637–644. 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6
Storkebaum E, Quaegebeur A, Vikkula M, Carmeliet P: Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat Neurosci 2011, 14: 1390–1397. 10.1038/nn.2947
Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA: Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 2000, 60: 55–69. 10.1006/mvre.2000.2244
Acknowledgements
We thank Alexandra Maria Lee for help with proofreading the manuscript and Edward Visse for assistance with drawing Figure 1.
Our work is supported by the Swedish Medical Research Council, the Anérs Foundation, and J.B. is supported by a grant from the European Union (FP7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IÖ, JB and GP wrote the manuscript. GP made the design for Figure1. IÖ contributed the tables and figure legends. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Özen, I., Boix, J. & Paul, G. Perivascular mesenchymal stem cells in the adult human brain: a future target for neuroregeneration?. Clin Trans Med 1, 30 (2012). https://doi.org/10.1186/2001-1326-1-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2001-1326-1-30