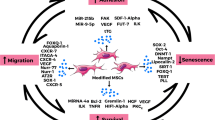
Abstract
Due to their multi-lineage differentiation capacity, support of haematopoiesis, immunomodulation and secretion of proregenerative factors, mesenchymal stem/stromal cells (MSCs) are in the focus of intense research since decades. The literature is replete with reports on their potential in preclinical model systems. However, the heterogeneity of the primary cell population as starting material and the diverse protocols for isolation and cultivation are hampering progress in their clinical application. Consensus on common standards and harmonised isolation and characterisation protocols are important to ensure safety and efficacy. This review focuses on the recent scientific evidence of clinically relevant properties and on the speculative cardiomyogenic and hepatic differentiation potential of MSCs. Special emphasis is put on the importance of standardisation and harmonisation in clinical-scale manufacturing.



Similar content being viewed by others
References
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9:641–650
Salem HK, Thiemermann C (2010) Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28:585–596
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Bernardo ME, Pagliara D, Locatelli F (2011) Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transplant 47:164–171
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Bieback K, Brinkmann I (2010) Mesenchymal stromal cells from human perinatal tissues: from biology to cell therapy. World J Stem Cells 2:81–92
Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W et al (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33:1402–1416
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Giordano A, Galderisi U, Marino IR (2007) From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol 211:27–35
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM (2000) Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 18:307–316
Philippe B, Luc S, Valerie PB, Jerome R, Alessandra BR, Louis C (2010) Culture and use of mesenchymal stromal cells in phase I and II clinical trials. Stem Cells Int 2010:503593
Bieback K, Kinzebach S, Karagianni M (2011) Translating research into clinical scale manufacturing of mesenchymal stromal cells. Stem Cells Int 2010:193519
Bauer-Kreisel P, Goepferich A, Blunk T (2010) Cell-delivery therapeutics for adipose tissue regeneration. Adv Drug Deliv Rev 62:798–813
Zhang X, Zhang C, Tang T, Qu Z, Lou J, Dai K (2008) Immunomodulatory and osteogenic differentiation effects of mesenchymal stem cells by adenovirus-mediated coexpression of CTLA4Ig and BMP2. J Orthop Res 26:314–321
Lee SJ, Kang SW, Do HJ, Han I, Shin DA, Kim JH, Lee SH (2010) Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 31:5652–5659
Brey D, Motlekar N, Diamond S, Mauck R, Garino J, Burdick J (2011) High-throughput screening of a small molecule library for promoters and inhibitors of mesenchymal stem cell osteogenic differentiation. Biotechnol Bioeng 108:163–237
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834
Cook MM, Futrega K, Osiecki M, Kabiri M, Kul B, Rice A, Atkinson K, Brooke G, Doran M (2012) Micromarrows-three-dimensional coculture of hematopoietic stem cells and mesenchymal stromal cells. Tissue Eng Part C Methods 18:319–328
Jing D, Fonseca AV, Alakel N, Fierro FA, Muller K, Bornhauser M, Ehninger G, Corbeil D, Ordemann R (2010) Hematopoietic stem cells in co-culture with mesenchymal stromal cells—modeling the niche compartments in vitro. Haematologica 95:542–550
Wagner W, Saffrich R, Wirkner U, Eckstein V, Blake J, Ansorge A, Schwager C, Wein F, Miesala K, Ansorge W et al (2005) Hematopoietic progenitor cells and cellular microenvironment: behavioral and molecular changes upon interaction. Stem Cells 23:1180–1191
Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R, Eckstein V, Ho AD (2007) Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells 25:2638–2647
Wagner W, Wein F, Roderburg C, Saffrich R, Faber A, Krause U, Schubert M, Benes V, Eckstein V, Maul H et al (2007) Adhesion of hematopoietic progenitor cells to human mesenchymal stem cells as a model for cell–cell interaction. Exp Hematol 35:314–325
Walenda T, Bork S, Horn P, Wein F, Saffrich R, Diehlmann A, Eckstein V, Ho AD, Wagner W (2010) Co-culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J Cell Mol Med 14:337–350
Wuchter P, Boda-Heggemann J, Straub BK, Grund C, Kuhn C, Krause U, Seckinger A, Peitsch WK, Spring H, Ho AD et al (2007) Processus and recessus adhaerentes: giant adherens cell junction systems connect and attract human mesenchymal stem cells. Cell Tissue Res 328:499–514
Ho AD, Wagner W, Franke W (2008) Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy 10:320–330
Wuchter P, Saffrich R, Wagner W, Wein F, Schubert MS, Eckstein V, Ho AD (2008) Human hematopoietic stem cells and leukemic cells form cadherin-catenin based junctional complexes with mesenchymal stromal cells. Blood 112:491
Wuchter P, Saffrich R, Giselbrecht S, Ho AD, Gottwald E (2011) Novel 3D-model for the hematopoietic stem cell niche using MSC in a KITChip based bioreactor. Blood (ASH Annual Meeting Abstracts) 118:1331
Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O (2003) Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57:11–20
Krampera M (2011) Mesenchymal stromal cell 'licensing': a multistep process. Leukemia 25:1408–1414
Krampera M (2011) Mesenchymal stromal cells: more than inhibitory cells. Leukemia 25:565–566
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726–736
Tyndall A, Pistoia V (2009) Mesenchymal stem cells combat sepsis. Nat Med 15:18–20
Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM (2010) A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 5:e10088
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822
Le Blanc K, Ringden O (2005) Use of mesenchymal stem cells for the prevention of immune complications of hematopoietic stem cell transplantation. Haematologica 90:438
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M et al (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586
Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M (2009) Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 52:79–86
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O et al (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67:1187–1194
Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, Yu Z, Li B, Xu C, Li Y et al (2008) The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 22:593–599
Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F 3rd (2011) Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells 29:11–19
Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9:11–15
Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AH, Lee MS, Lee MK, Kwon CH, Joh JW et al (2010) Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation 89:509–517
Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS et al (2005) Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11:367–368
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109:1543–1549
Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI (2010) Defining human mesenchymal stem cell efficacy in vivo. J Inflamm (Lond) 7:51
Fatar M, Stroick M, Griebe M, Marwedel I, Kern S, Bieback K, Giesel FL, Zechmann C, Kreisel S, Vollmar F et al (2008) Lipoaspirate-derived adult mesenchymal stem cells improve functional outcome during intracerebral hemorrhage by proliferation of endogenous progenitor cells stem cells in intracerebral hemorrhages. Neurosci Lett 443:174–178
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y (2005) Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309:314–317
Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H et al (1999) Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103:697–705
Choi YH, Kurtz A, Stamm C (2011) Mesenchymal stem cells for cardiac cell therapy. Hum Gene Ther 22:3–17
Rickelt S (2012) Plakophilin-2: a cell-cell adhesion plaque molecule of selective and fundamental importance in cardiac functions and tumor cell growth. Cell Tissue Res. doi:10.1007/s00441-011-1314-3
Borrmann CM, Grund C, Kuhn C, Hofmann I, Pieperhoff S, Franke WW (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol 85:469–485
Franke WW, Borrmann CM, Grund C, Pieperhoff S (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol 85:69–82
Franke WW, Schumacher H, Borrmann CM, Grund C, Winter-Simanowski S, Schlechter T, Pieperhoff S, Hofmann I (2007) The area composita of adhering junctions connecting heart muscle cells of vertebrates. III. Assembly and disintegration of intercalated disks in rat cardiomyocytes growing in culture. Eur J Cell Biol 86:127–142
Moll R, Holzhausen HJ, Mennel HD, Kuhn C, Baumann R, Taege C, Franke WW (2006) The cardiac isoform of alpha-actin in regenerating and atrophic skeletal muscle, myopathies and rhabdomyomatous tumors: an immunohistochemical study using monoclonal antibodies. Virchows Arch 449:175–191
Pieperhoff S, Borrmann C, Grund C, Barth M, Rizzo S, Franke WW (2010) The area composita of adhering junctions connecting heart muscle cells of vertebrates. VII. The different types of lateral junctions between the special cardiomyocytes of the conduction system of ovine and bovine hearts. Eur J Cell Biol 89:365–378
Pieperhoff S, Franke WW (2007) The area composita of adhering junctions connecting heart muscle cells of vertebrates. IV. Coalescence and amalgamation of desmosomal and adhaerens junction components—late processes in mammalian heart development. Eur J Cell Biol 86:377–391
Pieperhoff S, Schumacher H, Franke WW (2008) The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol 87:399–411
Seeger TS, Frank D, Rohr C, Will R, Just S, Grund C, Lyon R, Luedde M, Koegl M, Sheikh F et al (2010) Myozap, a novel intercalated disc protein, activates serum response factor-dependent signaling and is required to maintain cardiac function in vivo. Circ Res 106:880–890
Gaebel R, Furlani D, Sorg H, Polchow B, Frank J, Bieback K, Wang W, Klopsch C, Ong LL, Li W et al (2011) Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS One 6:e15652
Choi YH, Nasseri B, Stamm C (2011) Cardiac cell therapy and bypass surgery. Curr Pharm Des 17:3348–3355
Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD (2004) Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 363:783–784
Christ B, Dollinger MM (2011) The generation of hepatocytes from mesenchymal stem cells and engraftment into the liver. Curr Opin Org Transplant 16:69–75
Seo MJ, Suh SY, Bae YC, Jung JS (2005) Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun 328:258–264
Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE et al (2009) Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 58:570–581
Zemel R, Bachmetov L, Ad-El D, Abraham A, Tur-Kaspa R (2009) Expression of liver-specific markers in naive adipose-derived mesenchymal stem cells. Liver Int 29:1326–1337
Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M et al (2005) Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 106:756–763
Straub BK, Rickelt S, Zimbelmann R, Grund C, Kuhn C, Iken M, Ott M, Schirmacher P, Franke WW (2011) E-N-cadherin heterodimers define novel adherens junctions connecting endoderm-derived cells. J Cell Biol 195:873–887
Haridass D, Yuan Q, Becker PD, Cantz T, Iken M, Rothe M, Narain N, Bock M, Norder M, Legrand N et al (2009) Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am J Pathol 175:1483–1492
Mohamadnejad M, Pournasr B, Bagheri M, Aghdami N, Shahsavani M, Hosseini LA, Taghiabadi E, Azizi H, Heidari I, Akhlaghpoor S et al (2010) Transplantation of allogeneic bone marrow mesenchymal stromal cell-derived hepatocyte-like cells in homozygous familial hypercholesterolemia. Cytotherapy 12:566–568
Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, Tsuruyama T, Uemoto S, Kobayashi E (2011) Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One 6:e19195
van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML (2008) Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 47:1634–1643
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146
Bieback K, Schallmoser K, Klüter H, Strunk D (2008) Clinical protocols for the isolation and expansion of mesenchymal stromal cells. Transfus Med Hemother 35:286–294
Shibata K, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M, Kohno Y, Ito K, Fujibayashi S, Neo M et al (2007) Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem cells (Dayton, Ohio) 25:2371–2453
Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V et al (2008) Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3:e2213
Bieback K, Hecker A, Schlechter T, Hofmann I, Brousos N, Redmer T, Besser D, Klüter H, Müller AM, Becker M (2012) Replicative aging and differentiation potential of human adipose tissue-derived mesenchymal stromal cells expanded in pooled human or fetal bovine serum. Cytotherapy 14:570–583
Wagner W, Feldmann RE Jr, Seckinger A, Maurer MH, Wein F, Blake J, Krause U, Kalenka A, Burgers HF, Saffrich R et al (2006) The heterogeneity of human mesenchymal stem cell preparations—evidence from simultaneous analysis of proteomes and transcriptomes. Exp Hematol 34:536–548
Wagner W, Ho AD (2007) Mesenchymal stem cell preparations—comparing apples and oranges. Stem Cell Rev 3:239–248
Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M et al (2010) Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115:1549–1553
Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ et al (2011) Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 20:1297–1308
Mannello F, Tonti GA (2007) Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells 25:1603–1609
Bieback K, Ha VA, Hecker A, Grassl M, Kinzebach S, Solz H, Sticht C, Klüter H, Bugert P (2010) Altered gene expression in human adipose stem cells cultured with fetal bovine serum compared to human supplements. Tissue Eng Part A 16:3467–3484
Bieback K, Hecker A, Kocaomer A, Lannert H, Schallmoser K, Strunk D, Klüter H (2009) Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 27:2331–2341
Kocaoemer A, Kern S, Klüter H, Bieback K (2007) Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25:1270–1278
Perez-Simon JA, Lopez-Villar O, Andreu EJ, Rifon J, Muntion S, Campelo MD, Sanchez-Guijo FM, Martinez C, Valcarcel D, Canizo CD (2011) Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica 96:1072–1076
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD (2011) Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134:1790–1807
von Bonin M, Stolzel F, Goedecke A, Richter K, Wuschek N, Holig K, Platzbecker U, Illmer T, Schaich M, Schetelig J et al (2009) Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant 43:245–251
Acknowledgements
This work was supported by research funds of the German Federal Ministry of Education and Research (START-MSC 1 and 2: 01GN0531 and 01GN0939). We would like to thank Daniela Griffiths for proofreading.
Author disclosure statement
No competing financial interests exist.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Bieback, K., Wuchter, P., Besser, D. et al. Mesenchymal stromal cells (MSCs): science and f(r)iction. J Mol Med 90, 773–782 (2012). https://doi.org/10.1007/s00109-012-0915-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0915-y