Abstract
Background
A solid-state anaerobic digestion method is used to produce biogas from various solid wastes in China but the efficiency of methane production requires constant improvement. The diversity and abundance of relevant microorganisms play important roles in methanogenesis of biomass. The next-generation high-throughput pyrosequencing platform (Roche/454 GS FLX Titanium) provides a powerful tool for the discovery of novel microbes within the biogas-generating microbial communities.
Results
To improve the power of our metagenomic analysis, we first evaluated five different protocols for extracting total DNA from biogas-producing mesophilic solid-state fermentation materials and then chose two high-quality protocols for a full-scale analysis. The characterization of both sequencing reads and assembled contigs revealed that the most prevalent microbes of the fermentation materials are derived from Clostridiales (Firmicutes), which contribute to degrading both protein and cellulose. Other important bacterial species for decomposing fat and carbohydrate are Bacilli, Gammaproteobacteria, and Bacteroidetes (belonging to Firmicutes, Proteobacteria, and Bacteroidetes, respectively). The dominant bacterial species are from six genera: Clostridium, Aminobacterium, Psychrobacter, Anaerococcus, Syntrophomonas, and Bacteroides. Among them, abundant Psychrobacter species, which produce low temperature-adaptive lipases, and Anaerococcus species, which have weak fermentation capabilities, were identified for the first time in biogas fermentation. Archaea, represented by genera Methanosarcina, Methanosaeta and Methanoculleus of Euryarchaeota, constitute only a small fraction of the entire microbial community. The most abundant archaeal species include Methanosarcina barkeri fusaro, Methanoculleus marisnigri JR1, and Methanosaeta theromphila, and all are involved in both acetotrophic and hydrogenotrophic methanogenesis.
Conclusions
The identification of new bacterial genera and species involved in biogas production provides insights into novel designs of solid-state fermentation under mesophilic or low-temperature conditions.
Similar content being viewed by others
Background
Due to fossil fuel crisis, atmospheric pollution, and global warming, the development of renewable and clean energy forms has become a critical task for the human society. The production of biogas through biomass fermentation, regarded as an environment-friendly, clean, and renewable resource, has been gaining more attention in many developed and developing countries [1, 2]. In China, solid biomass wastes (SW), such as kitchen, livestock, and agricultural wastes (largely crop straws and stalks), are produced at the multi-million ton level annually [3] and the untreated disposals of such wastes may lead to severe long-term environmental hazards and resource wasting. Therefore, the utilization of anaerobic fermentation to convert SW into biogas represents a promising effort if it can be accomplished at an industrial scale and in an economical way. In recent years, solid-state anaerobic digestion (SS-AD) has been promoted in China because of its many advantages, including less reactor-capacity demand, lower heating-energy need, and no stirring-energy consumption, particularly as opposed to liquid-state anaerobic digestion [2]. However, the yield of methane, the major end-product of this process, has not been sufficient for an industrial-scale promotion, let alone economical plausibility.
The biochemical process for anaerobic methane production is complex. The diversity and abundance of microbes involved in the process certainly play a major role, which are influenced by microbial community compositions, fermentation materials, climate variations, and designs of chambers, to name just a few. In the initial steps of SS-AD, hydrolytic Firmicutes reduce large macromolecules (including proteins, complex fats, and polycarbohydrates) to their building blocks (i.e., amino acids, long-chain fatty acids, and monosugars) and other bacteria (including acidogens and acetogens) further degrade them into smaller intermediates (such as acetate, carbon dioxide, and hydrogen). In the later steps, methanogens, which are mainly derived from Archaea, convert the smaller substrates into methane through both aceticlastic and hydrogenotrophic pathways [4–6]. Therefore, a thorough understanding of composition, structure, and function of the microbial communities residing in anaerobic reactors is crucial for developing novel fermentation strategies and improving methane yield of the existing biogas reactors as well as ideas for novel designs.
Although the biochemistry and enzymology of methanogenesis for model organisms are well characterized [7], the structure and function of biogas-producing microbial communities have not been sufficiently explored, particularly under different anaerobic fermentation conditions. In the past decade or so, investigations of different biogas-producing systems and waste treatment conditions, including anaerobic mesophilic sludge digester [8], mesophilic anaerobic chemostats fed with synthetic wastewaters [9, 10], thermophilic upflow anaerobic filter reactor [11], fully- stirred reactor fed with fodder beet silage [12], thermophilic municipal biogas plant [13], and two-phase liquid biogas reactor operated with silages [14], have been conducted. Thermophilic anaerobic municipal solid-waste digester [15] and packed-bed reactor for degrading organic solid wastes of artificial garbage slurry [16] were also studied. However, the methodology used for these studies was based on constructing and sequencing 16S rDNA and mcrA clone libraries, and the choice of PCR primers for amplifying sequence fragments of the target genes and other sequences typically creates biases, and it has been difficult to cover the entire complexity of microbial communities based on just the sequences from a limited number of gene-specific clones. The next-generation sequencing technologies have overcome many of these problems, particularly the pyrosequencing platform (such as the Roche/454 GS FLX sequencer) that generates longer read lengths ranging from 200 to 400 bp as compared to other platforms (such as the Illumina Hiseq2000 system that generates 50–150 bp reads in its single-directional sequencing runs [17–20]) and creates less bias in sequencing library construction [21, 22]. Based on this platform, a German group conducted the first metagenomic analysis on a complex system of biogas-producing plant [20], and developed related bioinformatic methods [23, 24]. They further revealed that in addition to the archaeal methanogen Methanoculleus species (which play a dominant role in methane production) and abundant numbers of cellulolytic Clostridia (which were important for the hydrolysis of cellulosic plant biomass for acetogenesis) other methanogen taxa (including Streptococcus, Acetivibrio, Garciella, Tissierella, and Gelria) are also detected but their precise functional roles in methane formation remain to be elucidated [17, 25]. A similar study that used a SOLiD™ short-read DNA sequencing platform has recently confirmed the importance of hydrogen metabolism in biogas production [26]. Nevertheless, a metagenomic study on the SS-AD system based on deep-sampling and long-read sequencing supported by the next-generation sequencing platforms is of essence in moving the field forward.
Aside from sequencing and bioinformatic analysis, DNA extraction and its quality yield from samples of complex materials (such as liquid vs. solid and source vs. processing) also greatly affect results of metagenomic sequencing [27, 28]. DNA extraction efficiency and quality from biogas samples have also been compared to PCR-based analyses [29], but a robust method, particularly for analyzing samples from SW biogas fermentation materials and based on high-throughput shotgun pyrosequencing, has yet to be reported. Toward this end, we first evaluated five DNA extraction protocols (including four based on commercial kits and one derived from the classic chloroform-isoamylalcohol method) for samples collected from a mesophilic SS-AD fermenter fed with SW. After the T-RFLP evaluation (see Methods for details), we then chose the two better protocols and prepared DNA samples for our pyrosequencing-based metagenomic study. Our results have led to novel insights into microorganism composition, gene content, and metabolic capacity of the SW fermentation.
Results and discussion
Evaluation of DNA extraction methods for high-throughput pyrosequencing
Biogas fermentation samples are extremely complex due to the presence of multiple organic compounds and diverse degradation products. In SS-AD samples, microorganisms bind strongly to solid materials and have a rather heterogeneous distribution inside the samples. In order to find a better protocol for the isolation of high-quality DNA preparations for pyrosequencing, we set out to evaluate five DNA extraction methods. Using electrophoresis assay for checking quality and yield of genomic DNA extracts (Figure 1 and Table 1), we found that Protocols E, EY, and F gave rise to the highest yields, ranging from ~160.5 ng/μl to ~121.4 ng/μl, while Protocol P produced the lowest yield, with ~20.5 ng/μl. However, Protocol F showed the highest degree of smearing (Figure 1) and both Protocols F and S showed low purity based on A260/A230 ratios (Table 1). The DNA extract from Protocol S appeared dark yellowish and its quality could not be measured based on spectrophotometry. The differences among the five methods were primarily observed at the cell lysing steps, which are critical for DNA yield and quality especially when field sampling is the only source [29, 30]. According to our results, Protocol P (the Mo-Bio PowerSoil DNA Isolation Kit) showed the lowest DNA yield, suggesting insufficient lysing despite the use of vigorous mechanical force (vortexing for 15 min), especially when compared with the corresponding steps in other related protocols (hand shaking for a few minutes or vortexing for 30 s). Therefore, we realized that in addition to mechanical forces, lysis reagents used for the protocols may also be crucial for preparing better cell lysis.
The electrophoresis results of DNA preparations based on the five methods from a biogas reactor sample. M, molecular marker Transplus 2 K. P, F, E, EY, S refer to the five protocols, respectively. Except for Protocol P, which was loaded with 5 μl of undiluted DNA solution, only 1 μl of undiluted DNA solution was loaded on the 0.8% agarose gels for the rest of the extracts.
We further evaluated the DNA preparations from all five methods based on T-RFLP analysis. The Shannon-Weiner index was used to indicate diversity and complexity, and the Simpson index was used to measure abundance. Bacteria and archaea were analyzed separately. The results showed that Protocol E resulted in the highest bacterial diversity (Shannon-Weiner index of 3.6) and the highest abundance (Simpson index of 0.95; Table 2), followed by Protocols P and EY. Protocols E and EY showed higher archaeal enrichment than that of Protocols P, F and S. We therefore chose DNA extracts from Protocols E and EY for pyrosequencing, which consistently lead to higher yield, purer DNA, and high microbial diversity.
Sequencing and metagenomic assembly
Pyrosequencing of two DNA libraries (from Protocols E and EY), namely “BE” and “BEY”, were performed and the data from the experiments were summarized in Table 3. The first sequencing runs of BE (named as BE-1) and BEY resulted in 266,781,751 bp sequences from 738,005 reads (an average read length of 362 bp) and 197,514,392 bp sequences from 551,339 reads (an average read length of 358 bp), respectively. It is obvious that there are more data and higher microbial richness (Figure 2A in section 3.3) obtained from BE (BE-1) than from BEY. Therefore, the BE sample was sequenced twice again as BE-2 and BE-3.
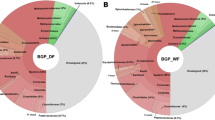
Rarefaction curves (A) and the relative proportions of taxonomic classification of bacteria, archaea, eukaryota, and viruses (B) from the BE-1 and BEY libraries. For the rarefaction curves, the analysis was performed on the total (including bacteria, archaea, eukaryota, viruses and environmental sequences), archaeal, and bacterial taxa from the two libraries.
Since the BE sample was sequenced three times, it yielded 647,369,218 bp sequences from 2,280,601 reads (in an average read length of 283 bp). The assembly of the total reads gave rise to 118,433 contigs containing 76,759,543 bp, which were accounted for approximately 12% of the total sequences measured in basepairs generated in this study. The number of large contigs (>500 nt) was 37,276 (an N50 of 1,712 bp), in which the largest contig contains 158,075 bp. The average GC content of the total reads from the BE sample is 46% (Additional file 1: Figure S1).
Comparison of microbial compositions between samples BE-1 and BEY
We used rarefaction analysis to assess species richness of the system. Using MEGAN (a meta-genomic bioinformatic tool) and at the best resolved levels based on the NCBI taxonomy database and our sequence data, we analyzed the microbial richness, based on sequence reads, between libraries BE-1 and BEY (Figure 2A) and revealed that the number of taxonomic leaves or clades of BE-1 are all higher than those of BEY, and the result indicated that BE-1 contains more microbial taxa than BEY, and indeed BE-1 and BEY contain 717 and 643 leaves for all assigned taxa, respectively. Furthermore, the rarefaction curves of both libraries in archaea appear close to saturation at 20% of the total reads, whereas those in bacteria are increased to 100% of the total reads. Our results suggest that the current sampling depth is not yet close to the natural status for bacteria but may be saturated for archaea.
Matching the sequencing reads from BE-1 and BEY to sequences collected in NT and NR databases, we dissected microbial community structure of the two libraries (excluding the reads with no-hits; Figure 2B), showing that at the domain level there is significant difference between the two libraries in the proportion of reads assigned to bacterial, archaeal, viral, and eukaryotic sequences (Figure 2B). In the BE-1 data set, 4.7% and 90.9% of the reads were assigned to archaea and bacteria, but decreased to 3.0% and 71.2% for those of BEY, respectively. In contrast, only 3.4% of the reads were assigned to eukaryotes and almost no viral sequence was detectable in BE-1, but eukaryotic and viral detections were significantly increased to 20.5% and 9.3% in BEY, respectively. The taxonomic bias in the microbial communities detected between the datasets from Protocols E and EY may reflect the thoroughness of sample pre-washing with TENP buffer that may partially wash off bacteria known to be lightly adhered to solid matrix.
Examining the taxonomies built from mapped single reads of libraries BE-1 and BEY (Additional file 1: Figure S2), we observed that the dominant taxa at the genus level for archaea and bacteria (such as Methanosarcina and Clostridium for the former and the latter, respectively) are comparable between the two libraries; but there were greater numbers of microbial taxa observed in the BE (combining BE-1, BY-2, and BE-3) and BEY libraries (Table 2 and Figure 2A). Therefore, Protocol E in combination with the E.Z.N.A.TM Soil DNA Kit should be considered as the most suitable procedure for the SW fermentation samples.
Microbial composition analysis based on sequencing reads and assembled contigs
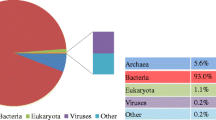
We analyzed the microbial community composition of the BE library using MEGAN and mapped individual reads first. Figure 3 shows the statistics of the total assigned reads and their annotations for the popular genera and phyla. The reads assigned to the superkingdoms Bacteria (~9.8%) and Archaea (~0.5%) were accounted for approximately 10.3% of the total reads, whereas 88.4% of the total reads obtained have no hit in the present database, indicating that there are still an immense amount of unknown/uncultured species in this complex anaerobic biogas-producing sample (Figure 3A).
The statistics for the reads assigned (A), the number of reads/contigs for the 11 most prevalent phyla (B), and the number of reads/contigs of 15 most prevalent genera in bacteria and the 3 most prevalent genera in archaea (C) obtained in the taxonomic classification analysis using BLASTN/BLASTX tools against the GenBank NT/NR database with a E-value cutoff of 10 -5 based on total reads/contigs.
Based on single-read assignments (Figure 3B), the most prevalent bacterial taxa at the phylum level are Firmicutes (39.0% of hit-reads), followed by Proteobacteria (17.3% of hit-reads) and Bacteroidetes (7.0% of hit-reads), which are responsible for biomass degradation and fermentation. The 4th most abundant taxon is Euryarchaeota (4.3% of hit-reads), involved in methane synthesis and taking a small fraction of the community. In addition, massive bacterial taxa are distributed in phyla Thermotogae (2.4% of hit-reads), Actinobacteria (2.2% of hit reads), Chloroflexi (1.2% of hit-reads), Cyanobacteria (0.4% of hit-reads), Chlorobi (0.3% of hit-reads), and Fusobacteria (0.3% of hit-reads), and the result again indicates that there are more complex microbial components residing within this system and that some of the components may reflect their original environments rather than characteristic of the SW feeds in general.
At the class level, the prevalent reads are distributed over Clostridia (66,455 reads, 27.2% of hit-reads), Bacilli (16,767reads, 6.9% of hit-reads), Gammaproteobacteria (19,085 reads, 7.8% of hit-reads), Bacteroidetes (11,765 reads, 4.8% of hit-reads), and Methanomicrobia (9180 reads, 3.8% of hit-reads), which belong to phyla Firmicutes, Proteobacteria, Bacteroidetes, and Euryarchaeota, respectively (Table 4).
At the genus level, there are 429 genera of bacterial and 39 genera of archaeal origins. The six most prevalent genera for bacteria are Clostridium (17,975 reads, 7.4% of hit-reads), Aminobacterium (11,870 reads, 5.2% of hit-reads), Psychrobacter (7,823 reads, 4.9% of hit-reads), Anaerococcus (6,544 reads, 2.7% of hit-reads), Bacteroides (5,655 reads, 2.3% of hit-reads), and Syntrophomonas (4698 reads, 1.9% of hit-reads). For archaeal species, the three most prevalent genera are Methanosarcina (6,522 reads, 2.7%), Methanoculleus (1,102 reads, 0.5%), and Methanosaeta (750 reads, 0.3%), which all belong to class Methanomicrobia of Euryarchaeota (Table 4, Figure 3C).
At the species level (Figure 4), although Clostridium is the predominant genus, the three most abundant bacterial species are Aminobacterium colombiense DSM 12261 (11,868 reads, 5.2%), Anaerococcus prevotii DSM 20548 (6,507 reads, 2.9%), Syntrophomonas wolfei subsp. Wolfei str. Goettingen (4,685 reads, 1.9%), while the dominant Clostridium species were identified as C. thermocellum ATCC 27405 (4,204 reads, 1.7%), C. tetani E88 (1,378 reads, 0.6%), C. kluyveri (1,100 reads. 0.5%), and C. phytofermentans ISDg (607 reads, 0.3%). In archaea, the three most prevalent species are Methanosarcina barkeri str. Fusaro (2,611 reads, 1.1%), Methanoculleus marisnigri JR1 (1,101 reads, 0.5%), Methanosaeta theromphila PT (724 reads, 0.3%).
Meanwhile, we also analyzed microbial community compositions based on the assembled contigs, with BLASTN (version 2.2.13) against the NT and NR databases with E-value cutoff of 10-5. Among a total of 118,433 contigs, 26,332 of them (22.2%) were assigned to 356 taxa (genus), containing 330 bacterial genera and 26 archaeal genera. Comparable to the taxonomic structure generated from the output of BLAST based on reads, our analysis showed that Firmicutes (32.2%), followed by Proteobacteria (14.1%), Bacteroidetes (3.8%), and Euryarchaeota (5.5%), are most dominant (Figure 3B, Additional file 1: Table S1). The dominant classes in bacteria are Clostridia (8,249 contigs), Gammaproteobacteria (1,972 contigs), Bacilli (484 contigs), Bacteroidetes (315 contigs), and those in archaea were mapped to Methanomicrobia (1,279 contigs). The 17 most prevalent genera and the 6 most prevalent species are also consistent with the taxonomic structure based on mapped reads (Figure 3C, Additional file 1: Table S1).
Since 16S rDNA is widely used for taxonomic and phylogenetic studies due to its highly conserved sequences in both bacteria and archaea and its hypervariable region can also be used for accurate taxonomic evaluation, we extracted 793 contigs (only 0.7% of total contigs) that contain 16S rDNA sequences (an average length of 1,068 bp) for further analysis. When submitted to the RDP database (with 80% confidence), approximately 68.6% and 1.3% of them were classified into bacteria and archaea, respectively. At the class level, the dominant taxa include Clostridia, Anaerolineae, Synergistia, Methanomicrobia, Bacilli, and Gammaproteobacteria (Additional file 1: Table S2), mostly from Firmicutes, Proteobacteria and Euryarchaeota. It is noteworthy that the detection of classes Anaerolineae and Synergistia to be the dominant taxa based on 16S rDNA sequences differs from those based on reads and contigs. The reasons are more complex. One of them may be information loss in short contigs and sequence assemblies of low-abundance species that are difficult to annotate based on limited matches. Another may be due to the lower matching rate for the 16S-associated contigs, where only approximately 7% of the contigs were classified at the genus level. Therefore, an even lower number of 16S rDNA genes was detected and assigned to the profile with significant certainty.
Novel anaerococcus and psychrobacterand their characteristics
We identified abundant reads assigned to two novel bacterial genera in the fermentation, and among these, the genus Anaerococcus of class Clostridia was identified, which was represented by the second most abundant bacterial species, A. prevotii DSM 20548 (also as A. prevotii PC1, Peptococcus prevotii) (6,507 reads, 2.7%), an obligate anaerobic bacterium. A. prevotii often presents in oral cavity, skin, vagina, gut [31], and deep-seated soft tissue, causing abscesses or anaerobic infections in humans [32]. Thus far, in Anaerococcus, only this strain has been completely sequenced due to its clinical significance, and the hit-reads for Anaerococcus were all assigned to this strain. Therefore, at the species level, the taxonomic prediction should be treated with caution. Our taxonomic assignment depends on the comparison of amino acid sequences deduced from reads encoding protein sequences of known taxonomic origin. Therefore, only previously sequenced species can be identified and there are possibilities that other Anaerococcus species do exist in the fermenter but conformations from further studies are inevitable.
According to the literature, most species in Anaerococcus are capable of fermenting several carbohydrates, although the fermentation power is weak [33]. However, the involvement of abundant Anaerococcus species in anaerobic fermentation has never been reported. The function of abundant Anaerococcus species in the fermentation remains unknown. Its existence in the fermentation may be contaminations from kitchen wastes or variability of the genus itself; although genetically identical, the species possess significant discrepancies and are subject to adaptation under certain fermentation conditions. In this study, several important enzymes in the metabolic pathways for methane synthesis were detected in association with Anaerococcus based on the results of the KEGG analysis (Figure 5, Additional file 1: Table S3), such as ackA (acetate kinase, EC: 2.7.2.1), pta (phosphate acetyltransferase, EC: 2.3.1.8) and transporters/antiporters, including NhaC and V-type ATPase subunit D (EC: 3.6.3.14), indicating that Anaerococcus species likely contribute to the methane synthesis in the fermentation. Therefore, it is necessary to isolate Anaerococcus species, to characterize their phylogenetic relationships, and to study their biological and ecological functions in SS-AD and methanogenesis from SW in future studies.
Methanogenesis pathway predicted in our fermenter based on the KEGG analysis. The ellipses denote the substances involved in the reaction. The boxes show the enzymes involved in methanogenesis. The acetotrophic (green), the hydrogenotrophic (blue), and the shared pathways (grey) are all color-encoded and the color depth indicates the amount of reads assigned. The enzymes that are potentially associated with Anaerococcus and Psychrobacter in the methanogenesis pathway are shown in the yellow box.
The genus Psychrobacter (7,823 reads, 3.2%) of class Gammaproteobacteria (phylum Proteobacteria) is primarily comprised of P. cryohalolentis (1,431 reads, 0.6%), P. arcticus (425 reads, 0.2%), and Psychrobacter sp. PRwf-1 (524 reads, 0.2%). Most species of this genus can adapt to cold conditions, such as polar permafrost and ice, and are capable of reproducing at temperatures ranging from −10°C to 40°C [34]. The species can even be found in the relatively thermophilic environment of the digestion conditions. So far, Psychrobacter sp. have been defined as aerobic and mesophilic bacteria. However, some researchers have shown that some of their strains were also able to grow in fermenting environments [35–37] and anaerobic conditions, such as facultative anaerobic bacteria [38]. The Psychrobacter species often produce variable lipases (including phenylalanine deaminase, alkaline phosphatase, esterase (C4), esterase lipase (C8), lipase (C14), leucine arylamidase, and lecithinase [39, 40]) and play essential roles in fat decomposition reactions. They have been isolated from the facial and body tissues of animals, poultry carcass, fermented seafood [38, 41–43], and groundwater, but the isolation of Psychrobacter species has never been reported in biogas fermentation samples. In this study, several important enzymes in methane metabolism pathways associated with this genus were also detected based on the KEGG analyses(Figure 5, Additional file 1: Table S3), including ackA (acetate kinase, EC: 2.7.2.1), pta (phosphate acetyltransferase, EC: 2.3.1.8), acetyl-CoA synthetase (EC: 6.2.1.1) and transporters/antiporters (such as NhaA and NhaC), and the results indicate that these enzymes most likely participate in fat hydrolysis in SW samples for methanogenesis, particularly under mesophilic conditions.
Fat hydrolysis is the primary reaction of lipases. Moreover, lipases catalyze esterification, interesterification, acidolysis, alcoholysis and aminolysis reactions in addition to the hydrolytic activity on triglycerides [44]. Therefore, cold-active lipases, largely distributed in psychrophilic microorganisms and showing high catalytic activity at low temperatures, are added to detergents for cold washing, industrial food fermentation samples, environmental bioremediation (digesters, composting, oil or xenobiotic biology applications) and biotransformation processes [44]. Some cold-adaptive lipase genes from Psychrobacter sp. had been previously cloned and expressed [45]. Therefore, the abundance of Psychrobacter sp. in this fermenter demonstrates great potential for use in SW treatment for methane production or in other bio-energy conversion processes based on fatty-rich substrates, particularly under low temperature conditions, in northern China.
Other dominant bacterial species and their characteristics
The most frequently predicted species in this fermenter is A. colombiense DSM 12261 (11,868 reads, 5.1%), primarily isolated from anaerobic sludge and belongs to genus Aminobacterium (Clostridia) [46]. A. colombiense DSM 12261 and the relative species of Aminobacterium are both syntrophic, capable of anaerobic degradation of amino acids, particularly without saccharides and consistently identified in anaerobic environment, such as sludge and compost [46, 9]. The abundance of such amino acid-metabolizing organisms indicates high protein content in the SW samples.
S. wolfei subsp. Wolfei str. Goettingen (4,685 reads, 1.9%) belongs to genus Syntrophomonas (Clostridia), often isolated from anaerobic environments, such as aquatic sediment or sewage sludge, growing together with methanogens (such as Methanospirillum hungatii) and other H2-using and/or formate-using microorganisms [47]. S. wolfei subsp. Wolfei str. Goettingen participates in anaerobic fatty acid degradation [48] through the degradation of long-chain fatty acids into acetate and H2[49] due to the activity of acyl-CoA dehydrogenase, CoA transferase, enoyl-CoA hydratase, and other related enzymes [48], and this species plays a significant role in fatty acid decomposition and methanogenesis.
The reads for genus Bacteroides (5,655 reads, 2.1%) were mainly assigned to B. thetaiotaomicron VPI-5482 (1,406 reads, 0.6%), B. vulgatus ATCC 8482 (1,402 reads, 0.6%), and B. fragilis YCH46 (183 reads, 0.1%). Bacteroides often reside in human and animal intestines so that they exhibit symbiotic relationship with E. coli and other species. Bacteroides are involved in the fermentation of dietary polysaccharides, utilization of nitrogenous substances, and biotransformation of bile acids and other steroids in human colon [50]. Most intestinal bacteria are saccharolytic and obtain carbon and energy through hydrolysis of carbohydrates.
The dominant Clostridium species was identified as C. thermocellum (4,204 reads, 1.7%), which directly converts cellulosic substrate into ethanol with high efficiency and is a good candidate for the degradation of cellulosic materials from plant biomasses [20, 51–53]. In addition to plants, some Clostridium species can also be isolated from animal feces and cultured with Methanobacterium thermoautotrophicum[51]. The tetanus-causing bacterium C. tetani (1,378 reads, 0.6%) is an obligate anaerobe that relies on fermentation. It can be found in manure-treated soil, animal feces, and fermentation samples from biogas-producing plant [20]. C. kluyveri (1,100 reads. 0.5%) grows anaerobically, using ethanol and acetate as sole energy sources to produce butyrate, caproate, and H2[54]. C. kluyveri is originally identified from canal mud [55] and C. phytofermentans (607 reads, 0.3%) is widely distributed in soil, capable of producing ethanol, acetate, CO2, and H2 through fermenting cellulose [56]. Therefore, in the initial steps of biomass digestion, members of Clostridium produce a wide variety of extracellular enzymes to degrade large biological molecules (such as cellulose, xylans, proteins, and lipids into fermentable components) [6] and participate in acetogenesis—the pathway prior to methanogenesis—to create precursors for methane production [17].
Thermanaerovibrio acidaminovorans DSM 6589 (1,948 reads, 0.8%) (also known as strain Su883) is a thermophilic anaerobic but air-tolerance organism [57]. This species is versatile, grows on a variety of amino acids, and can be co-cultured with Methanobacterium thermoautotrophicum Z245 to improve methane production [58]. The presence of this species in the sample suggests amino acid accumulation through protein and polypeptide degradation, and it is also evidenced by the fact that there are many genes assigned to the category of “amino acid transport and metabolism” in the COG and KEGG annotations (Figure 6).
Categorization of the biogas-fermenter metagenomic sequencing reads according to the Clusters of Orthologous Groups of proteins (COGs). The categories are abbreviated as follows: J, translation, ribosomal structure and biogenesis; A, RNA processing and modification; K, transcription; L, replication, recombination and repair; B, chromatin structure and dynamics; D, cell cycle control, cell division, chromosome partitioning; V, defense mechanisms; T, signal transduction mechanisms; M, cell wall/membrane/envelope biogenesis; N, cell motility; W, extracellular structures; U, intracellular trafficking, secretion, and vesicular transport; O, posttranslational modification, protein turnover, chaperones; C, energy production and conversion; G, carbohydrate transport and metabolism; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolites biosynthesis, transport and catabolism; R, general function prediction only; and S, function unknown.
Dominant archaeal species and their characteristics
The most prevalent archaeal species was assigned to Methanosarcina barkeri fusaro (2,611 reads, 1.1%), which is originally isolated from sediment obtained from Lago del Fusaro, a freshwater coastal lagoon of West Naples, Italy [59]. This reference species possesses a relatively thick cell wall (composed of acidic heteropolysaccharide) that forms a protective sheath, and it uses versatile substrates for methane synthesis, making it more adaptable to the environment as compared with its relatives. In addition to its strong survival ability, M. barkeri is capable of raising pH level in the surrounding area [60]. Similar to landfill, solid-state fermentation also accumulates acids produced by acetogens that make the environment too acidic to host methanogens. However, attributable to the M. barkeri accumulation, a lesser acidic environment can be maintained in the fermenter and other methanogens can benefit from it. In addition, this microbe often makes trash mound more compact and creates more room for waste treatment [60].
The genus Methanosarcina includes many methanogens whose metabolic features are diverse and include both acetotrophic and hydrogenotrophic pathways. In particular, some strains in this genus are capable of utilizing methanol [59]. Furthermore, most Methanosarcina are immotile and mesophilic, exhibiting multiple metabolic features with strong advantage in survival. It is proposed that methanol is one of the major factors that influence methanogenesis [61]. In SW treatments, there are approximately 60% of the total mass containing complex organic matters and products of hydrolysis and acidogenesis are most likely multiple since members of order Methanosarcinales have the widest substrate range among methanogens [62]. The dominance of Methanosarcina demonstrates the relatively abundant nutrient sources and various metabolic pathways within our fermentation system.
The second dominant archaeal taxon is Methanoculleus marisinigri JR1 (1,101 reads, 0.5%), an organism that belongs to order Methanomirobiales and class Methanomicrobia. This species is capable of producing methane through the reduction of CO2 with H2 and uses formate and secondary alcohols as alternative electron donors sometimes, i.e., the hydrogenotrophic pathway. However, M. marisinigri JR1 cannot use acetate and methyl group-containing compounds for methanogenesis, i.e., the acetotrophic pathway. M. marisinigri JR1 is relatively small in cell dimensions and grows under moderate conditions with temperature ranging from 10°C to 32°C and pH ranging from 6.8 to 7.3. M. marisinigri JR1 is found in both thermophilic anaerobic digester [63] and the leachate of a full-scale recirculating landfill [64]. Particularly, in a metagenomic study of methanogens residing in a biogas-producing plant in Germany, M. marisinigri JR1 is found being the most abundant species in the reactor [20].
The third abundant archaeal methanogen was identified as Methanosaeta theromphila (724 reads, 0.3%), a member of Methanosaeta, which is the only genus of family Methanosaetaceae. M. theromphila are non-motile, non-sporulating, and thermophilic, which thrives at temperature of 50°C or higher, though it only grows at near neutral pH. M. theromphila is rod-shaped and capable of producing acetate kinase that activates acetate to acetyl-CoA in the first step of fermenting acetate to methane [65].
Gene function annotation and classification
To obtain a metabolic profile for this discrete bacterial community, we annotated all sequences (total reads) using BLASTX based on COG and KEGG database (Figure 6). Approximately 28% of the total reads were assigned to one or more COG functional categories. In the category “metabolism”, a large amount of reads are distributed among “carbohydrate transport and metabolism (G)”, “amino acid transport and metabolism (E)”, “energy production and conversion (C)” and “lipid transport and metabolism (I)” (Additional file 1: Figure S3). These metabolic activities are associated with the conversion of biomass into methane during anaerobic fermentation. In the KEGG analysis, metabolism terms, including purine, pyrimidine, amino sugar and nucleotide sugar, glycolysis/gluconeogenesis and methane metabolisms are among the top five most popular categories (Additional file 1: Figure S4). Many of these metabolic processes are involved in the conversion of carbohydrates to simple compounds and the use of methane in the absence of oxygen. For example, pyruvate:ferredoxin oxidoreductase and related 2-oxoacid:ferredoxin oxidoreductases (COG0674, 2217 reads), Glycosidases (COG0366, 2632 reads), nucleoside-diphosphate-sugar epimerases (COG0451, 2219 reads), sugar permeases (COG0395, 2050 reads), and glucan phosphorylase (COG0058, 1211 reads) were all inevitably detected in this system.
The enzymes involved in carbohydrate metabolism were detected in reads assigned to “amino and nucleotide sugar metabolisms (4,217 reads)”, “glycolysis/gluconeogenesis (4,212 reads)” and “starch and sucrose metabolisms (3,170 reads)” as the three most dominant groups, which are involved in processing of monosaccharides and polyose, such as maltase-glucoamylase [EC: 3.2.1.20], beta-glucosidase [EC: 3.2.1.21], glycogen-debranching enzyme [EC: 2.4.1.25 3.2.1.33], levansucrase [EC: 2.4.1.10], chitinase [EC: 3.2.1.14], and glucokinase [EC: 2.7.1.2]. This observation is consistent with the finding that many species in this fermentation sample are involved in carbohydrate digestion and energy conversion.
There are also abundant reads that matched to genes for “lipid metabolism” (4,385 reads), such as fatty acid, glycerolipid, glyceropholipid, arachidonic acid, and linoleic acid metabolisms. Many of the enzymes detected in the processes, such as dihydroxyacetone kinase [EC: 2.7.1.29], glycerate kinase [EC: 2.7.1.31], glycerol-3-phosphate dehydrogenase (NAD(P)+) [EC: 1.1.1.94], glycerol-3-phosphate dehydrogenase [EC: 1.1.5.3] and acetyl-CoA acyltransferase [EC: 2.3.1.16], are also involved in methane metabolism. In addition, a significant amount of reads were obtained for the processes involved in the protein degradation pathway (1,820 reads), such as ATP-dependent Clp protease proteolytic subunit [EC: 3.4.21.92] and ATP-dependent protease La [EC: 3.4.21.53], EC: ATP-dependent protease HsIV [EC: 3.4.25]. Approximately 15% (v/v) of the kitchen waste in our fermenter contain both fat and protein, and both lipid hydrolysis and peptide degradation provide fermentation substrates for the downstream methanogenesis.
Moreover, total contigs with lengths longer than 500 bp were also analyzed against the KEGG database based on the BLAST tools. Non-eukaryotic contigs ranging from 10 to 60 kbp were extracted from the BLAST output files, and the contigs with identities lower than 80% or with alignment lengths shorter than 100 bp were filtered out. For each contig, we selected the best-hit sequences based on the highest score. The functional annotations of the large contigs (Additional file 1: Table S4) showed that there are 16 contigs with hits to genetic information processing pathway, 12 contigs for environmental information processing, 9 contigs for cellular processes, and 8 contigs for nucleotide metabolism. For amino acid, carbohydrate, and energy metabolism, the numbers of large contigs with best-hits were 4, 4, and 3, respectively. Larger contig suggests higher sequencing-read coverage. Therefore, the abundant microorganisms are always the active participants in the degradation of organic materials and energy exchange under the fermentation conditions.
Metabolic pathway analysis in the SW fermentation
The two distinct methanogenic pathways are from H2/CO2 to methane (hydrogenotrophic pathway) and from acetate to methane (acetotrophic pathway). Methanogenesis has also been shown to use carbon from other small organic compounds, such as formate, methanol, methylamines, dimethyl sulfide, and methanethiol, which are usually classified intermediates or substrates of the H2/CO2-to-methane pathway. Figure 5 shows the elements of the two methanogenesis pathways detected in our study. Many large contigs (20 contigs in a total length of 76,331 bp; Additional file 1: Table S3), such as contig17513 (10,585 bp) for the formate dehydrogenase (EC: 1.2.1.2) and contig06034 (10,805 bp) for the formylmethanofuran dehydrogenase (fwdA/fmdA, EC: 1.2.99.5), which are involved in the initial step of the hydrogenotrophic pathway (Figure 5, blue box), were detected in the sample. In addition, mtd, mer (EC: 1.5.99.9), frhB (EC: 1.12.98.1), ftr (EC: 2.3.1.101) and mch (EC: 3.5.4.27), which are also involved in the hydrogenotrophic pathway, were present. Moreover, a significant number of contigs (17 contigs, total length 38,078 bp, in Additional file 1: Table S3) were mapped to the acetotrophic pathway (Figure 5, green boxes). In this pathway, acetyl-CoA synthetase (acs, EC: 6.2.1.1) plays a key role in the synthesis of acetyl-CoA from acetate. Acetyl-CoA synthetase is involved in the acetyl-CoA decarbonylase/synthase complex (ACDS; composed of CdhA1, CdhB, CdhD, CdhE and CdhC) and cleaves C-C/C-S bonds in the acetyl moiety of acetyl-CoA to oxidize the carbonyl group into CO2 and to transfer the methyl group to tetrahydrosarcinapterin.
Methanogens use 2-mercaptoethanesulfonate (CoM; coenzyme M) as the terminal methyl carrier in methanogenesis. Tetrahydromethanopterin S-methyltransferase (mtr, EC: 2.1.1.86), methyl coenzyme M reductase (mcr, EC: 2.8.4.1) and reductase heterodisulfide reductase (Hdr, EC: 1.8.98.1), which are required for the final reaction steps of both methanogenic pathway, were also identified in our sample (Figure 5 and Additional file 1: Table S3). Furthermore, the finding of critical enzymes, such as phosphosulfolactate synthasein (coma, EC: 4.4.1.19), 2-phosphosulfolactate phosphatase (comb, EC: 3.1.3.71), and (R)-2-hydroxyacid dehydrogenase (EC: 1.1.1.272), for coenzyme M biosynthesis (data not shown in Figure 5 but in Additional file 1: Table S3) provides insights into the SW fermentation process. Moreover, our pathway analyses defined a variety of transporters/antiporters involved in the methanogenic pathways, such V-type H + −transporting ATPase and Na+:H + antiporter (nha) (not shown in Figure 5 but in Additional file 1: Table S3). Therefore, both hydrogenotrophic and acetotrophic pathways for methanogenesis occur almost equally in our fermenter, and the conclusion is strongly supported by the evidence from our data and consistent with the metabolic characteristics of the dominant archaeal species and complex components of the microbial communities in the SW fermentation.
Conclusions
Using high-throughput pyrosequencing and optimized DNA extraction protocols, we characterized microbial communities of mesophilic SS-AD fermentation and their related metabolic pathways in biomass degradation and methane synthesis. First, we aligned the reads and assembled contigs separately to the related databases and found that bacteria and archaea took 91.5% and 4.4% of the hits from the sequencing reads, respectively. Members from Firmicutes, Clostridia and Bacilli, are mostly enriched, followed by phyla Proteobacteria and Bacteroidetes. Particularly, the species from genera Aminobacterium, Psychrobacter, Anaerococcus, Clostridium, Syntrophomonas, and Bacteroides play key roles in the initial degradation of protein, fat, cellulose, and other polysaccharides. These results were further supported by gene functional annotation where we detected many enzymes involved in “protein degradation”, “lipid metabolism”, and “carbohydrate metabolism”.
Second, the dominant methanogens present in this fermenter were from Methanomicrobia. The most prevalent species appears to be Methanosarcina barkeri fusaro, which uses versatile substrates and contains both acetotrophic and hydrogenotrophic pathways for methane synthesis [62]. M. marisinigri JR1 and M. theromphila with either hydrogenotrophic or acetotrophic pathways for methanogenesis appear less abundant.
Third, the Psychrobacter (class Gammaproteobacteria) and Anaerococcus (class Clostridia) species are obviously abundant in the fermenter, but they have seldom been reported in other biogas fermentation samples. The Psychrobacter species adapt to extremely cold climates and produce cold-adaptive lipases [34] and have great potential to be used in low-temperature fermentation, particularly in northern China. However, Anaerococcus species exhibit weak fermentation capability [33] but abundant in SS-AD, playing roles in biomass degradation efficiency and methane yield. Our findings indicate that it is important to identify these species and to characterize them for their ecological and biological functions under SS-AD conditions, particularly for the rational design of microbial community structures to improve biogas production in solid-state fermentation under low-temperature conditions.
Methods
Sample preparation for DNA extraction
The samples for total DNA extraction were obtained from an anaerobic digester with a 2-liter working capacity. The digester was loaded with multi-component substrates, including kitchen waste (15%, v/v over the total solid added), pig manure (42.5%) and excess sludge (42.5%), and the initial total solid content was 20% (v/v for the total container volume). The anaerobic digestion was operated at 35 ± 1°C. The samples were collected from the digester when biogas production entered a steady phase. On the sampling day, the biogas yield was 72% biomethane at pH 7.0.
Total DNA extraction
The liquid content of samples (0.25 g fresh weight) was removed by centrifugation at 13,000 rounds per minute (rpm) for 10 min at 4°C. Subsequently, five different protocols (Protocols E, EY, F, P, and S) were used to extract total DNA according to the manufacturer’s instructions and laboratory manuals. 30-μl double distilled (dd) H2O were used to dissolve the DNA at the final step regardless what stated in the various protocols.
Protocol E: the E.Z.N.A.TM Soil DNA Kit (Omega Bio-Tek, Inc., USA) was used with minor modifications. Briefly, in the lysis step, vortexing was replaced by hand shaking for approximately 10 min to dissolve the pellet.
Protocol EY: The sample (0.25 g) was washed twice with 1.5 ml of TENP buffer [66], vortexed for 10 min, collected through centrifugation (12000 rpm, 5 min), neutralized with 1 ml of PBS buffer, and subjected to Protocol E for DNA extraction.
Protocol F: the FastDNA Spin Kit (for soil DNA extraction, MP Biomedicals, Heidelberg, Germany) was used with small adjustment in the lysis step as in Protocol E. In the purification step, the Spin Filter was washed twice with 500 μl SEWS-M buffer for better DNA purity.
Protocol P: the Mo-Bio PowerSoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA) was used with minor modifications. The original lysis time was changed to 15 min with maximum intensity, and the sample was centrifuged for longer time (12000 rpm, 2 min) to completely degrade cell walls. In the purification step, the Spin Filter was washed twice with 500 μl of solution C5 for better DNA purity.
Protocol S: the sample (0.25 g) was pre-washed as done in Protocol EY before DNA extraction according to modified method of Zhou et al. (1996) [67]. Briefly, after adding 0.25 g glass beads (d = 1 mm) and 0.75 ml DNA extraction buffer (100 mM Tris, 100 mM EDTA, 200 mM NaCl, 0.01 g/ml PVP, 2% CTAB, pH = 8.0) to the pretreated pellet, the sample was vortexed for 5 min. Subsequently, 0.75 ml SDS buffer (100 mM Tris, 200 mM NaCl, 2% SDS, pH = 8) was added and mixed with hand-shaking for 5 min. The sample was incubated at 65°C for 10 min and inverted every 10 min for a total of 5 times. After centrifugation at 12000 rpm for 15 min at room temperature, the middle-layer liquid was collected, extracted with an equal volume of chloroform-isoamyl alcohol (24:1, v/v), precipitated with isopropanol, and washed with 70% ethanol.
DNA quantification
The total DNA yield and quality were determined spectrophotometrically (NanoDrop 3300, Thermo Fisher Scientific Inc. USA), followed by electrophoresis on 0.8% agarose gels.
T-RFLP analysis
The 16S rDNA was PCR amplified using the universal bacterial primer set containing 8 F-FAM (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5’- GGTTACCTTGTTACGACTT-3′) [68] and the archaeal domain-specific primer set containing Arc109F-FAM (5′-ACKGCTCAGTAACACGT-3′) and Arc 915R (5′-GTGCTCCCCCGCCAATTCCT-3′) [69], respectively. The 5′-ends of primers 8 F and Arc109F were labeled with 6-carboxyfluoresceinphosphoramidite (FAM). The PCR reactions were performed with an rTaq-polymerase (TAKARA biotechnology (Dalian) Co., Ltd., Japan.) for 25 cycles and the annealing temperature was 60°C for bacteria and 55°C for archaea. The PCR products were subsequently purified using the QIAquick PCR purification kit (QIAGEN China Co., Ltd., Germany), and a 50-μl aliquot of each PCR product was digested with the restriction enzymes MspI and TaqI (New England Biolabs (Beijing) Co., Ltd. USA), respectively, for 2 h and subjected to the gene scan analysis on an ABI 3730 DNA Analyzer at Shanghai GeneCore BioTechnologies Co., Ltd. China prior to terminal restriction fragment length polymorphism (T-RFLP) analysis.
Pyrosequencing of total DNA
Total DNA from fermentation samples was sheared and sized to produce DNA whole-genome-shotgun library according to the manufacturer’s protocol from GS FLX Titanium General Library Preparation Kit (Roche Applied Science, USA). DNA Sequencing was performed on a 454 GS FLX Titanium platform at the Beijing Institute of Genomics, Chinese Academy of Sciences.
Statistics of the biogas-metagenome sequencing data
The shotgun sequences were assembled by using the GS de novo assembler. Raw and statistical sequencing data were summarized according to the assembly output. Both raw reads and contigs were used for further analysis.
Classification of sequencing data
The classification of the total data was performed by using the BLASTN/BLASTX tools against GenBank NT/NR databases with an E-value cutoff of 10-5 based on total reads and contigs.
The species richness analysis was performed by using MEGAN based on total sequencing reads [70]. The MEGAN platform uses the lowest common ancestor (LCA) algorithm to classify reads to certain taxa based on their blast hits. The LCA parameters were set as Min Score 35.0, Top Percent 50, and Min Support 2.
In addition, the 16S rDNA contigs with hits were extracted from the results of BLASTN analysis against the NT database and submitted to the Ribosomal Database Project (RDP) database [71] for classification with 80% confidence.
A rarefaction curve was generated for all reads, except unassigned and no-hit reads. The results of the total read classification were constructed into a rooted taxonomic tree where each clade (leaf) represents a taxon. The clades (leaves) in this tree were subsequently used as operational taxonomic units (OTUs) in the rarefaction analysis. The program randomly and incrementally chooses a tenth of the reads as a subset until all the reads are chosen. For each random subset, the number of leaves is determined independently.
Functional annotation of total contigs
To obtain gene profile characteristic for the anaerobic microbial community, the total sequencing reads were annotated based on BLASTX analysis against the database of Clusters of Orthologous Groups of proteins (COG) [72] with an E-value cut-off of 10−5. The sequencing reads were functionally annotated and assigned to the COG categories according to their best hits.
The metabolism analysis was performed on KEGG Orthology (KO)-identifiers by using KAAS tool (KEGG Automatic Annotation Server) with bi-directional best hit of total contigs, a default threshold (60), and prokaryotes as a representative set. Gene annotation was based on Enzyme Commission (EC)-numbers based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology database. Metabolic pathway maps were drawn according to the list of unique EC numbers.
Authors’ information
S Li is a Professor, A Li is a M. Phil student, X Liu is an Associate Professor, and J Yan and L Zhang are postdocs of Institute of Nuclear and New Energy Technology, Tsinghua University, China.
J Yu is a Professor of the CAS Key Laboratory of Genome Sciences and Information, Beijing Institute of Genomics (BIG), Chinese Academy of Sciences (CAS), China. Y Chu is a PhD student, and X Wang, L Ren and S Wu are Associate Professors of BIG, CAS, China.
All abbreviations for enzymes and genes involved in the methanogenesis pathways in Figure 5 are listed in Additional file 1: Table S3.
Abbreviations
- SS-AD:
-
Solid state anaerobic digestion
- SW:
-
Solid biomass waste
- PBS buffer:
-
Phosphate buffered saline
- CTAB:
-
Cetyltrimethylammonium bromide
- EDTA:
-
Ethylene diamine tetraacetie acid
- PVP:
-
Polyvinylpyrrolidone
- SDS:
-
Sodium dodecyl sulfate
- rpm:
-
Rounds per minute
- FAM:
-
6-Carboxyfluoresceinphosphoramidite
- T-RFLP:
-
Terminal restriction fragment length polymorphism
- nt:
-
Nucleotide
- NT database:
-
Non-redundant nucleotide sequence database, with entries from all traditional divisions of GenBank EMBL, and DDBJ excluding bulk divisions (gss, sts, pat, est, htg divisions) and wgs entries
- NR database:
-
Non-redundant protein sequence database with entries from GenPept, swissprot, PIR, PDF, PDB, and RefSeq
- LCA:
-
Lowest common ancestor
- RDP database:
-
Ribosomal database project database
- OTUs:
-
Operational taxonomic units
- COG:
-
Clusters of orthologous groups of proteins
- KEEG:
-
Kyoto encyclopedia of genes and genomes orthology database
- KO:
-
KEGG ortholog
- KAAS:
-
KEGG automatic annotation server
- EC:
-
Enzyme commission
- dd H2O:
-
Double distilled H2O
- CoM:
-
Coenzyme M
- CoA:
-
Coenzyme A
- ACDS:
-
Acetyl-CoA decarbonylase/synthase complex
- NAD(P)+:
-
Nicotinamide adenine dinucleotide (phosphate)
- 16S rDNA:
-
16S rRNA gene sequence
- PCR:
-
Polymerase chain reaction
References
Weiland P: Production and energetic use of biogas from energy crops and wastes in Germany. Appl Biochem Biotech. 2003, 109: 263-274. 10.1385/ABAB:109:1-3:263.
Yadvika , Santosh , Sreekrishnan TR, Kohli S, Rana V: Enhancement of biogas production from solid substrates using different techniques--a review. Bioresour Technol. 2004, 95: 1-10. 10.1016/j.biortech.2004.02.010.
Liu X, Wang W, Gao X, Zhou Y, Shen R: Effect of thermal pretreatment on the physical and chemical properties of municipal biomass waste. Waste Manag. 2012, 32: 249-255. 10.1016/j.wasman.2011.09.027.
Demirel B, Scherer P: The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev Environ Sci Biotechnol. 2008, 7: 173-190. 10.1007/s11157-008-9131-1.
Ferry JG: Fundamentals of methanogenic pathways that are key to the biomethanation of complex biomass. Curr Opin Biotechnol. 2011, 22: 351-357. 10.1016/j.copbio.2011.04.011.
Bryan P, Tracy SWJ, Fast AG, Indurthi DC, Papoutsakis ET: Current opinion in biotechnology. Clostridia: the importance of their exceptionalsubstrate and metabolitediversity for biofuel and biorefineryapplications. 2011, Available online 11 November 2011
Schink B: Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol R. 1997, 61: 262-
Chouari R, Le Paslier D, Daegelen P, Ginestet P, Weissenbach J, Sghir A: Novel predominant archaeal and bacterial groups revealed by molecular analysis of an anaerobic sludge digester. Environ Microbiol. 2005, 7: 1104-1115. 10.1111/j.1462-2920.2005.00795.x.
Tang YQ, Shigematsu T, Morimura S, Kida K: Microbial community analysis of mesophilic anaerobic protein degradation process using bovine serum albumin (BSA)-fed continuous cultivation. J Biosci Bioeng. 2005, 99: 150-164. 10.1263/jbb.99.150.
Shigematsu T, Era S, Mizuno Y, Ninomiya K, Kamegawa Y, Morimura S, Kida K: Microbial community of a mesophilic propionate-degrading methanogenic consortium in chemostat cultivation analyzed based on 16S rRNA and acetate kinase genes. Appl Microbiol Biot. 2006, 72: 401-415. 10.1007/s00253-005-0275-4.
Tang YQ, Fujimura Y, Shigematsu T, Morimura S, Kida K: Anaerobic treatment performance and microbial population of thermophilic upflow anaerobic filter reactor treating awamori distillery wastewater. J Biosci Bioeng. 2007, 104: 281-287. 10.1263/jbb.104.281.
Klocke M, Mahnert P, Mundt K, Souidi K, Linke B: Microbial community analysis of a biogas-producing completely stirred tank reactor fed continuously with fodder beet silage as mono-substrate. Syst Appl Microbiol. 2007, 30: 139-151. 10.1016/j.syapm.2006.03.007.
Weiss A, Jerome V, Freitag R, Mayer HK: Diversity of the resident microbiota in a thermophilic municipal biogas plant. Appl Microbiol Biot. 2008, 81: 163-173. 10.1007/s00253-008-1717-6.
Klocke M, Nettmann E, Bergmann I, Mundt K, Souidi K, Mumme J, Linke B: Characterization of the methanogenic archaea within two-phase biogas reactor systems operated with plant biomass. Syst Appl Microbiol. 2008, 31: 190-205. 10.1016/j.syapm.2008.02.003.
Tang YQ, Shigematsu T, Ikbal , Morimura S, Kida K: The effects of micro-aeration on the phylogenetic diversity of microorganisms in a thermophilic anaerobic municipal solid-waste digester. Water Res. 2004, 38: 2537-2550. 10.1016/j.watres.2004.03.012.
Sasaki K, Haruta S, Ueno Y, Ishii M, Igarashi Y: Microbial population in the biomass adhering to supporting material in a packed-bed reactor degrading organic solid waste. Appl Microbiol Biot. 2007, 75: 941-952. 10.1007/s00253-007-0888-x.
Jaenicke S, Ander C, Bekel T, Bisdorf R, Droge M, Gartemann KH, Junemann S, Kaiser O, Krause L, Tille F: Comparative and joint analysis of two metagenomic datasets from a biogas fermenter obtained by 454-pyrosequencing. PLoS One. 2011, 6: e14519-10.1371/journal.pone.0014519.
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z: Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005, 437: 376-380.
Rothberg JM, Leamon JH: The development and impact of 454 sequencing. Nat Biotechnol. 2008, 26: 1117-1124. 10.1038/nbt1485.
Schluter A, Bekel T, Diaz NN, Dondrup M, Eichenlaub R, Gartemann KH, Krahn I, Krause L, Kromeke H, Kruse O: The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology. J Biotechnol. 2008, 136: 77-90. 10.1016/j.jbiotec.2008.05.008.
Simon C, Wiezer A, Strittmatter AW, Daniel R: Phylogenetic diversity and metabolic potential revealed in a glacier ice metagenome. Appl Environ Microb. 2009, 75: 7519-7526. 10.1128/AEM.00946-09.
Abbai NS, Govender A, Shaik R, Pillay B: Pyrosequence analysis of unamplified and whole genome amplified DNA from hydrocarbon-contaminated groundwater. Mol Biotechnol. 2012, 50: 39-48. 10.1007/s12033-011-9412-8.
Krause L, Diaz NN, Edwards RA, Gartemann KH, Kromeke H, Neuweger H, Puhler A, Runte KJ, Schluter A, Stoye J: Taxonomic composition and gene content of a methane-producing microbial community isolated from a biogas reactor. J Biotechnol. 2008, 136: 91-101. 10.1016/j.jbiotec.2008.06.003.
Krause L, Diaz NN, Goesmann A, Kelley S, Nattkemper TW, Rohwer F, Edwards RA, Stoye J: Phylogenetic classification of short environmental DNA fragments. Nucleic Acids Res. 2008, 36: 2230-2239. 10.1093/nar/gkn038.
Krober M, Bekel T, Diaz NN, Goesmann A, Jaenicke S, Krause L, Miller D, Runte KJ, Viehover P, Puhler A, Schluter A: Phylogenetic characterization of a biogas plant microbial community integrating clone library 16S-rDNA sequences and metagenome sequence data obtained by 454-pyrosequencing. J Biotechnol. 2009, 142: 38-49. 10.1016/j.jbiotec.2009.02.010.
Wirth R, Kovacs E, Maroti G, Bagi Z, Rakhely G, Kovacs KL: Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol Biofuels. 2012, 5: 41-56. 10.1186/1754-6834-5-41.
Desai N, Antonopoulos D, Gilbert JA, Glass EM, Meyer F: From genomics to metagenomics. Curr Opin Biotechnol. 2012, 23: 72-76. 10.1016/j.copbio.2011.12.017.
Scholz MB, Lo CC, Chain PS: Next generation sequencing and bioinformatic bottlenecks: the current state of metagenomic data analysis. Curr Opin Biotechnol. 2012, 23: 9-15. 10.1016/j.copbio.2011.11.013.
Bergmann I, Mundt K, Sontag M, Baumstark I, Nettmann E, Klocke M: Influence of DNA isolation on Q-PCR-based quantification of methanogenic archaea in biogas fermenters. Syst Appl Microbiol. 2010, 33: 78-84. 10.1016/j.syapm.2009.11.004.
Hwang C, Ling F, Andersen GL, LeChevallier MW, Liu WT: Evaluation of methods for the extraction of DNA from drinking water distribution system biofilms. Microbes and environments / JSME. 2012, 27: 9-18. 10.1264/jsme2.ME11132.
Ezaki T, Yamamoto N, Ninomiya K, Suzuki S, Yabuuchi E: Transfer of peptococcus-indolicus, peptococcus-asaccharolyticus, peptococcus-prevotii, and peptococcus-magnus to the genus peptostreptococcus and proposal of peptostreptococcus-tetradius Sp-Nov. Int J Syst Bacteriol. 1983, 33: 683-698. 10.1099/00207713-33-4-683.
Murdoch DA: Gram-positive anaerobic cocci. Clin Microbiol Rev. 1998, 11: 81-
Ezaki T, Kawamura Y, Li N, Li ZY, Zhao LC, Shu SE: Proposal of the genera anaerococcus gen. nov., peptoniphilus gen. nov and gallicola gen. nov for members of the genus peptostreptococcus. Int J Syst Evol Micr. 2001, 51: 1521-1528.
Rodrigues DF, Jesus ED, Ayala-del-Rio HL, Pellizari VH, Gilichinsky D, Sepulveda-Torres L, Tiedje JM: Biogeography of two cold-adapted genera: psychrobacter and exiguobacterium. ISME J. 2009, 3: 658-665. 10.1038/ismej.2009.25.
Yoon JH, Lee CH, Kang SJ, Oh TK: Psychrobacter celer sp nov., isolated from sea water of the south Sea in Korea. Int J Syst Evol Micr. 2005, 55: 1885-1890. 10.1099/ijs.0.63682-0.
Yoon JH, Lee CH, Yeo SH, Oh TK: Psychrobacter aquimaris sp nov and Psychrobacter namhaensis sp nov., isolated from sea water of the South Sea in Korea. Int J Syst Evol Micr. 2005, 55: 1007-1013. 10.1099/ijs.0.63464-0.
Jung SY, Lee MH, Oh TK, Park YH, Yoon JH, Park’ YH: Psychrobacter cibarius sp nov., isolated from jeotgal, a traditional Korean fermented seafood. Int J Syst Evol Micr. 2005, 55: 577-582. 10.1099/ijs.0.63398-0.
Liao VHC, Chu YJ, Su YC, Hsiao SY, Wei CC, Liu CW, Liao CM, Shen WC, Chang FJ: Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol. 2011, 123: 20-29. 10.1016/j.jconhyd.2010.12.003.
Yumoto I, Hirota K, Sogabe Y, Nodasaka Y, Yokota Y, Hoshino T: Psychrobacter okhotskensis sp nov., a lipase-producing facultative psychrophile isolated from the coast of the Okhotsk Sea. Int J Syst Evol Micr. 2003, 53: 1985-1989. 10.1099/ijs.0.02686-0.
Bozal N, Montes MJ, Tudela E, Guinea J: Characterization of several psychrobacter strains isolated from Antarctic environments and description of psychrobacter luti sp nov and psychrobacter fozii sp nov. Int J Syst Evol Micr. 2003, 53: 1093-1100. 10.1099/ijs.0.02457-0.
Kampfer P, Albrecht A, Buczolits S, Busse HJ: Psychrobacter faecalis sp nov., a new species from a bioaerosol originating from pigeon faeces. Syst Appl Microbiol. 2002, 25: 31-36. 10.1078/0723-2020-00099.
Vela AI, Collins MD, Latre MV, Mateos A, Moreno MA, Hutson R, Dominguez L, Fernandez-Garayzabal JF: Psychrobacter pulmonis sp. nov., isolated from the lungs of lambs. Int J Syst Evol Microbiol. 2003, 53: 415-419. 10.1099/ijs.0.02413-0.
Yoon JH, Kang KH, Park YH: Psychrobacter jeotgali sp. nov., isolated from jeotgal, a traditional Korean fermented seafood. Int J Syst Evol Microbiol. 2003, 53: 449-454. 10.1099/ijs.0.02242-0.
Joseph B, Ramteke PW, Thomas G: Cold active microbial lipases: Some hot issues and recent developments. Biotechnol Adv. 2008, 26: 457-470. 10.1016/j.biotechadv.2008.05.003.
Zhang J, Lin S, Zeng RY: Cloning, expression, and characterization of a cold-adapted lipase gene from an Antarctic deep-sea psychrotrophic bacterium, psychrobacter sp 7195. J Microbiol Biotechn. 2007, 17: 604-610.
Baena S, Fardeau ML, Labat M, Ollivier B, Thomas P, Garcia JL, Patel BKC: Aminobacterium colombiense gen. nov. sp. nov., an amino acid-degrading anaerobe isolated from anaerobic sludge. Anaerobe. 1998, 4: 241-250. 10.1006/anae.1998.0170.
Beaty PS, Wofford NQ, Mcinerney MJ: Separation of syntrophomonas-wolfei from methanospirillum-hungatii in syntrophic cocultures by using percoll gradients. Appl Environ Microb. 1987, 53: 1183-1185.
Wofford NQ, Beaty PS, Mcinerney MJ: Preparation of cell-free-extracts and the enzymes involved in fatty-acid metabolism in syntrophomonas-wolfei. J Bacteriol. 1986, 167: 179-185.
Mackie RI, Bryant MP: Metabolic-activity of fatty acid-oxidizing bacteria and the contribution of acetate, propionate, butyrate, and Co2 to methanogenesis in cattle waste at 40-degrees-C and 60-degrees-C. Appl Environ Microb. 1981, 41: 1363-1373.
Xu J, Chiang HC, Bjursell MK, Gordon JI: Message from a human gut symbiont: sensitivity is a prerequisite for sharing. Trends Microbiol. 2004, 12: 21-28. 10.1016/j.tim.2003.11.007.
Weimer PJ, Zeikus JG: Fermentation of cellulose and cellobiose by clostridium thermocellum in the absence of methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977, 33: 289-297.
Beguin P, Millet J, Aubert JP: Cellulose degradation by clostridium thermocellum: from manure to molecular biology. FEMS Microbiol Lett. 1992, 79: 523-528.
Sparling R, Islam R, Cicek N, Carere C, Chow H, Levin DB: Formate synthesis by clostridium thermocellum during anaerobic fermentation. Can J Microbiol. 2006, 52: 681-688. 10.1139/w06-021.
Seedorf H, Fricke WF, Veith B, Bruggemann H, Liesegang H, Strittimatter A, Miethke M, Buckel W, Hinderberger J, Li FL: The genome of clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc Natl Acad Sci U S A. 2008, 105: 2128-2133. 10.1073/pnas.0711093105.
Barker HA: The production of caproic and butyric acids by the methane fermentation of ethyl alcohol. Arch Microbiol. 1937, 8: 415-421.
Warnick TA: Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int J Syst Evol Micr. 2002, 52: 1155-1160. 10.1099/ijs.0.02125-0.
Baena S, Fardeau ML, Woo THS, Ollivier B, Labat M, Patel BKC: Phylogenetic relationships of three amino-acid-utilizing anaerobes, selenomonas acidaminovorans, ‘Selenomonas acidaminophila’ and eubacterium acidaminophilum, as inferred from partial 16S rDNA nucleotide sequences and proposal of thermanaerovibrio acidaminovorans gen. nov., comb. nov and anaeromusa acidaminophila gen. nov., comb. nov. Int J Syst Bacteriol. 1999, 49: 969-974. 10.1099/00207713-49-3-969.
Plugge CM, Stams AJM: Arginine catabolism by thermanaerovibrio acidaminovorans. FEMS Microbiol Lett. 2001, 195: 259-262. 10.1111/j.1574-6968.2001.tb10530.x.
Kandler O, Hippe H: Lack of peptidoglycan in the cell walls of methanosarcina barkeri. Arch Microbiol. 1977, 113: 57-60. 10.1007/BF00428580.
Staley BF, de Los Reyes FL, Barlaz MA: Effect of spatial differences in microbial activity, pH, and substrate levels on methanogenesis initiation in refuse. Appl Environ Microbiol. 2011, 77: 2381-2391. 10.1128/AEM.02349-10.
Maeder DL, Anderson I, Brettin TS, Bruce DC, Gilna P, Han CS, Lapidus A, Metcalf WW, Saunders E, Tapia R, Sowers KR: The methanosarcina barkeri genome: comparative analysis with methanosarcina acetivorans and methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J Bacteriol. 2006, 188: 7922-7931. 10.1128/JB.00810-06.
Liu Y: Methanosarcinales. Handbook of Hydrocarbon and Lipid Microbiology. Volume 1. Edited by: Timmis KN. 2010, Berlin Heidelberg: Springer-Verlag
Hori T, Haruta S, Ueno Y, Ishii M, Igarashi Y: Dynamic transition of a methanogenic population in response to the concentration of volatile fatty acids in a thermophilic anaerobic digester. Appl Environ Microb. 2006, 72: 1623-1630. 10.1128/AEM.72.2.1623-1630.2006.
Huang LN, Zhou H, Chen YQ, Luo S, Lan CY, Qu LH: Diversity and structure of the archaeal community in the leachate of a full-scale recirculating landfill as examined by direct 16S rRNA gene sequence retrieval. FEMS Microbiol Lett. 2002, 214: 235-240. 10.1111/j.1574-6968.2002.tb11353.x.
Singh-Wissmann K, Ingram-Smith C, Miles RD, Ferry JG: Identification of essential glutamates in the acetate kinase from methanosarcina thermophila. J Bacteriol. 1998, 180: 1129-1134.
Picard C, Ponsonnet C, Paget E, Nesme X, Simonet P: Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain-reaction. Appl Environ Microb. 1992, 58: 2717-2722.
Zhou JZ, Bruns MA, Tiedje JM: DNA recovery from soils of diverse composition. Appl Environ Microb. 1996, 62: 316-322.
DeLong EF, Preston CM, Mincer T, Rich V, Hallam SJ, Frigaard NU, Martinez A, Sullivan MB, Edwards R, Brito BR: Community genomics among stratified microbial assemblages in the ocean’s interior. Science. 2006, 311: 496-503. 10.1126/science.1120250.
Baker BJ, Banfield JF: Microbial communities in acid mine drainage. FEMS Microbiol Ecol. 2003, 44: 139-152. 10.1016/S0168-6496(03)00028-X.
Huson DH, Auch AF, Qi J, Schuster SC: MEGAN analysis of metagenomic data. Genome Res. 2007, 17: 377-386. 10.1101/gr.5969107.
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM: The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37: D141-D145. 10.1093/nar/gkn879.
Tatusov RL, Galperin MY, Natale DA, Koonin EV: The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28: 33-36. 10.1093/nar/28.1.33.
Acknowledgement
This research was funded by the Ministry of Science and Technology of China, the International Scientific and Technological cooperation project (Grant No. 2010DFB64040), 0061nd the National Science & Technology Pillar Program (Grant No. 2012BAC18B01). The CAS Key Laboratory of Genome Sciences and Information, Beijing Institute of Genomics, Chinese Academy of Sciences supported the data analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interest
The authors declare that were no competing interests.
Authors’ contributions
Study concept and design: SL, JY. Fermentation setting and sampling: AL, LZ. DNA extraction and evaluation: AL, YC. Bioinformatics analysis: YC, AL. Analysis and interpretation of data: YC, AL. Acquisition of data: SW, XW, LR, JY. Drafting of the manuscript: AL, YC, SW. Critical revision of the manuscript for important intellectual content: SL JY. Obtaining funding: SL, JY. Administrative, technical, or material support: XW, LR, SW, XL. Supervision: SL, JY. All the authors have read and approved the final manuscript.
An Li, Ya’nan Chu contributed equally to this work.
Electronic supplementary material
13068_2012_259_MOESM1_ESM.doc
Additional file 1: Table S1: Top 17 genera of taxonomic classification based on contig-counts. Table S2. Analysis of bacterial and archaeal 16S-rDNA contigs based on the Ribosomal Database Project Classifier (RDPC). Table S3. Large contig function annotation. Table S4. The list of contigs detected in methanogenesis pathways. Figure S1. The histogram shows the distribution of the GC percentage for BE-1 sample. Each position represents the number of sequences within a GC percentage range. The data used in these graphs is based on raw upload and post quality-control sequences. Figure S2. Comparison of microbial community structures between BE-1 and BEY. The taxonomic trees of BE-1 and BEY on rank family for archaea and on rank class for bacteria were constructed respectively on MEGAN. A, archaea of BE-1. B, archaea of BEY. C, bacteria of BE-1. D, bacteria of BEY. Figure S3. Popular terms in metabolism based on KEGG analysis. The Y-axis refers to the percentage of reads within the reads mapping to metabolism terms. Figure S4. Popular terms in the functional secondary category of metabolism based on KEGG analysis. (DOC 1278 kb) (DOC 1 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, A., Chu, Y., Wang, X. et al. A pyrosequencing-based metagenomic study of methane-producing microbial community in solid-state biogas reactor. Biotechnol Biofuels 6, 3 (2013). https://doi.org/10.1186/1754-6834-6-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1754-6834-6-3